-
Original Article10-23-2024
Access and adequacy of antenatal care in a city in Brazil during two phases of the COVID-19 pandemic
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo87
Abstract
Original ArticleAccess and adequacy of antenatal care in a city in Brazil during two phases of the COVID-19 pandemic
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo87
Views191Abstract
Objective:
To compare access and suitability of antenatal care between years 2020 and 2022 among postpartum individuals at a Hospital in Florianopolis, and evaluate factors associated with antenatal suitability.
Methods:
Observational, cross-sectional, and quantitative study carried out in 2022. Collected data were compared with the database of a previous similar study carried out in the same setting in 2020. Data were extracted from medical records and prenatal booklets, in addition to a face-to-face questionnaire. Adequacy was measured using the Carvalho and Novaes index and health access was qualitatively evaluated. Socio-demographic and antenatal variables were analyzed. A statistical significance level of 0.05 was considered. Open-ended questions were categorized for analysis.
Results:
395 postpartum individuals were included. Antenatal care was adequate for 48.6% in 2020 and 69.1% in 2022. Among the barriers to access, 56% reported difficulty in scheduling appointments and/or exams and 23% complained of reduced healthcare staff due to strikes, COVID-19, among others. Adequate antenatal care was associated with being pregnant in 2022, being referred to high-risk units (PNAR), and not reporting difficulties in access. Also, it was associated with twice the chance of investigation for gestational diabetes (GDM) and syphilis.
Conclusion:
The 2022 post-vaccination period showed higher antenatal adequacy. The main difficulty for postpartum individuals was scheduling appointments and/or exams. Having antenatal care in 2022, no reports of difficulty in access, and follow-up at a high-risk unit were associated with antenatal adequacy.
Key-words COVID-19Delivery of health careDiabetesGestationalpandemicsPostpartum periodPregnancyPrenatal caresurveys and questionnairesVaccinationSee more -
Original Article10-23-2024
The role of HIV as an independent risk factor to cervical HSIL recurrence
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo85
Abstract
Original ArticleThe role of HIV as an independent risk factor to cervical HSIL recurrence
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo85
Views264ABSTRACT
Objective:
To evaluate the role of being human immunodeficiency virus (HIV) positive for predicting the risk of recurrence in women with a cervical high grade squamous intraepithelial lesion (HSIL) diagnosis.
Methods:
Retrospective observational case-control study, comprising HIV positive (case) and HIV negative (control) women in a 1:4 ratio. Women assisted by the Erasto Gaertner Hospital, between 2009-2018, with cervical HSIL diagnosis, submitted to treatment by Loop electrosurgical excision procedure (LEEP), and with a minimum follow-up of 18 months, were included. The immunological status, number and time to recurrence were analyzed, with p<0.05 considered significant. In a second analysis, only patients with free margins were evaluated.
Results:
The sample consisted of 320 women (64 cases and 256 controls). Presence of HIV, CD4 levels <200 and detectable viral load (CV) were associated with high risk of recurrence, with odds ratio (OR) of 5.4 (p<0.001/95CI:2.8-10); 3.6 (p<0.001 /IC95:0.6-21.1) and 1.8 (p=0.039 /IC95:0.3-9.3), respectively. In the sample with free margins (n=271), this risk was also higher among seropositive patients, with OR 4.18 (p=0.001/95CI:1.8-9.2).
Conclusion:
HIV is an independent risk factor for cervical HSIL recurrence and reduced disease-free survival time. Glandular involvement, compromised margins, undetectable CV and CD4<200 also increase the risk of relapse.
Key-words Disease-free survivalElectrosurgeryExcision marginsHIV infectionsRecurrenceRisk factorsSquamous intraepithelial lesionsUterine cervical neoplasmsSee more -
Original Article10-23-2024
Assessment of risk factors associated with post-molar gestational trophoblastic neoplasia: a retrospective cohort
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo83
Abstract
Original ArticleAssessment of risk factors associated with post-molar gestational trophoblastic neoplasia: a retrospective cohort
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo83
Views157ABSTRACT
Objective:
Evaluate the risk factors for the development of post-molar gestational trophoblastic neoplasia.
Methods:
Retrospective cohort study with 320 women with gestational trophoblastic disease (GTD) followed in a tertiary hospital from January 2005 to January 2020. Data referring to the women’s sociodemographic profile, clinical, laboratory and treatment aspects and types of GTD were analyzed.
Results:
The mean age of women with the benign form was 26.4±8.6 years and with the malignant forms 26.9±8.5 years (p=0.536). Most women with malignant forms came from regions further away from reference center (p=0.012), had vesicle elimination at the time of diagnosis (p=0.028) and needed more than one uterine evacuation (p<0.001) when compared to the benign forms. There was no difference between laboratory tests in both forms. Being between 30 and 39 years old increased the chance of developing invasive mole by 2.5 (p=0.004; 95%CI:1.3–4.9) and coming from regions far from reference center by 4.01 (p=0.020; CI95%: 1.2-12.9). The women with the highest risk of malignant forms were those with the longest time of become normal on human gonadotrophic hormone (hCG) testing (each week the risk increases 1.3 times; p<0.001, 95%CI: 1.2-1.3).
Conclusion:
The prolonged hCG fall curve is the main indicator of an increased chance of GTN. Women from regions further away from reference center have a greater chance of developing malignant forms, probably due to the difficulty in accessing the reference center and, therefore, adequate follow-up that would allow early identification of more serious cases.
Key-words Gestational trophoblastic diseaseGestational trophoblastic neoplasmHydatidiform mole, invasiveSee more -
Original Article10-23-2024
Validation of Brazilian Version of the Sexual Desire Inventory 2 (SDI-2)
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo78
Abstract
Original ArticleValidation of Brazilian Version of the Sexual Desire Inventory 2 (SDI-2)
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo78
Views225ABSTRACT
Objective:
To traslate and validate of the Brazilian version of the SDI-2.
Methods:
This was a cross-sectional study. The cultural adaptation considered the stages of initial translation, synthesis of translations, evaluation by a committee of experts from different regions of Brazil, back-translation, and pre-test. The content validity and psychometric proprieties was assessed.
Results:
Ten specialists participated in the cultural adaptation of the SDI-2. The content validity showed a Content Validity Ratio (CVR) ≥ 0.75 (p = 0.05). A total of 674 subjects participated in the field study. The Exploratory Factorial Analysis (EFA) presented factor loads ≥ 0.445, and commonalities ≥ 0.40; and two dimensions represented 77% of the total variance explained. The Confirmatory Factorial Analysis CFA presented X2/df = 4.265; the Root Mean Square Error of Approximation RMSEA = 0.110; the Non-Normed Fit Index NNFI = 0.946; the Comparative Fit Index (CFI) = 0.963; the Goodness of Fit Index GFI = 0.986; and the Adjusted Goodness of Fit Index AGFI = 0.979 for a two-factor model. The coefficient values for the total SDI-2 score were 0.91 for Cronbach’s alpha, 0.91 for McDonald’s Omega, and 0.97 for the Greatest Lower Bound GLB coefficients. The invariance between sexes was 0.01 for the ΔCFI and ΔRMSEA, showing model stability for these two populations.
Conclusion:
The Brazilian version of the SDI-2 is self-report, valid, reliable and invariant across sex.
Key-words cross cultural comparisonLibidoPsychometricsSexual behaviorSexual desiresurveys and questionnairesSee more -
FEBRASGO POSITION STATEMENT10-15-2024
Immunization in women’s lives: present and future
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS10
Abstract
FEBRASGO POSITION STATEMENTImmunization in women’s lives: present and future
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS10
Views270See moreKey points
•The negative impact of infectious diseases and their immunoprevention during the different stages of a woman’s life requires a broad approach including adolescence, adulthood, pregnancy and the postmenopausal phase.
•Immunization of pregnant women should be a priority for the protection of the maternal-fetal dyad, especially in regions with high rates of infections preventable by immunization.
•Brazil has one of the most comprehensive vaccination programs in the world – the National Immunization Program (Programa Nacional de Imunizações, PNI) – that serves all age groups: newborns, children, adolescents, adults, pregnant women and older adults, as well as groups with special needs, such as adolescents, pregnant and older adult women.
•However, vaccination coverage remains below ideal for all available vaccines, especially among adolescents and pregnant women, and Febrasgo is committed to collaborating with the PNI to combat vaccine hesitancy.
•The gynecologist/obstetrician is the reference physician for women, therefore the access to information and updates regarding all vaccines recommended for their patients is extremely important for this professional, aiming at the greatest possible protection.
•The objective of this Febrasgo Position Statement is to bring an update to women’s vaccination schedule, covering some vaccines that are available, including new approved vaccines and those in the commercialization phase.
•This work is a compilation of the First Febrasgo Scientific Immunization Forum held in the city of São Paulo in October 2023 with the objective to update recommendations for vaccines in use and new innovative vaccines soon to be available.
-
GUIDELINES10-07-2024
Brazilian Guideline on Menopausal Cardiovascular Health – 2024
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo100
Abstract
GUIDELINESBrazilian Guideline on Menopausal Cardiovascular Health – 2024
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo100
Views2681. IntroductionAfter the publication of the results from the Women’s Health Initiative (WHI) clinical trial in 2002 showing more risks than benefits to female health with estrogen (alone or combined with progestin) use to control menopausal signs and symptoms, there has been a progressive and sustained decline in the prescription of those drugs.–In the United […]See more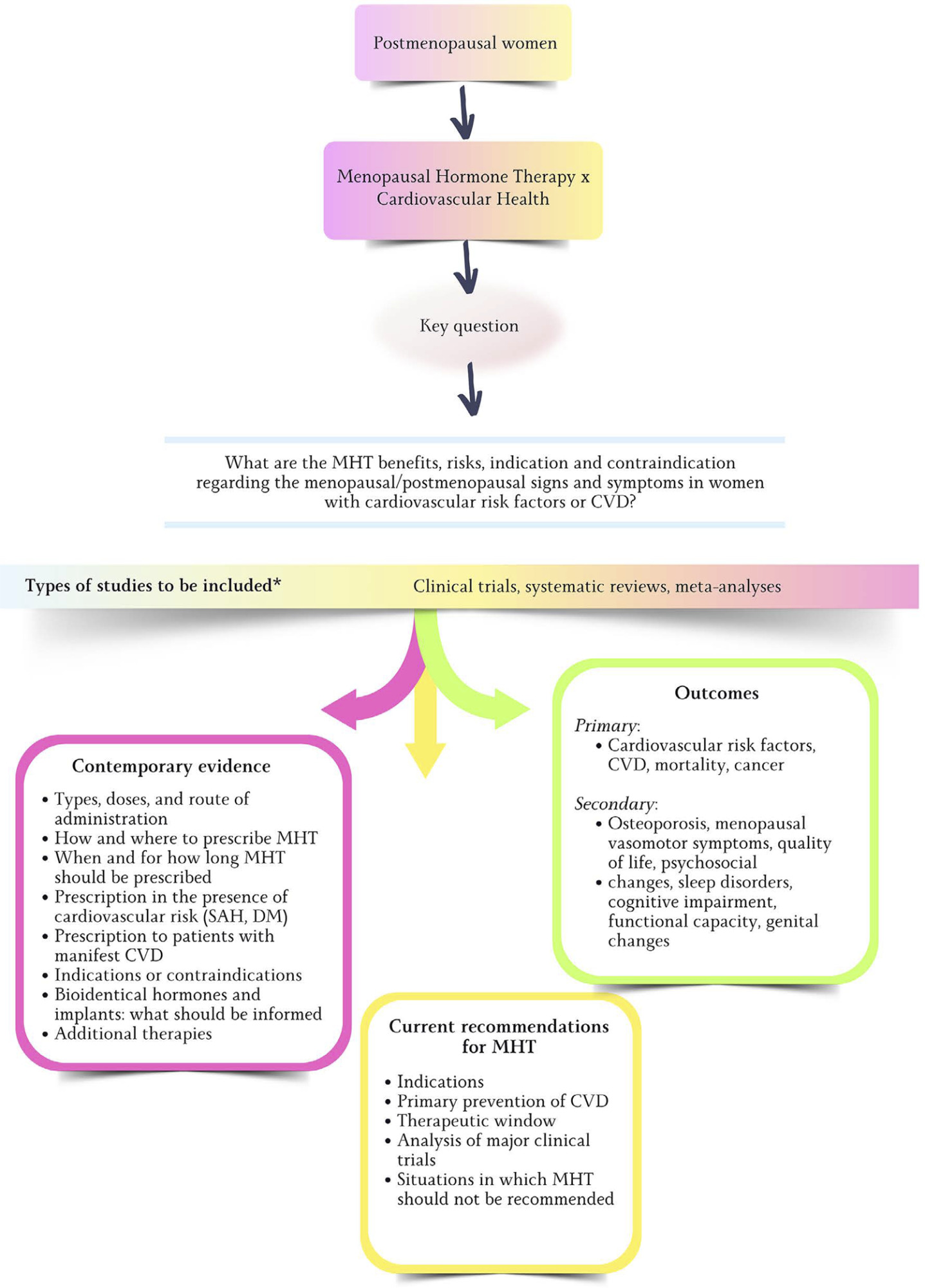
-
FEBRASGO POSITION STATEMENT09-23-2024
Syphilis and pregnancy
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS09
Abstract
FEBRASGO POSITION STATEMENTSyphilis and pregnancy
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS09
Views314See moreKey points
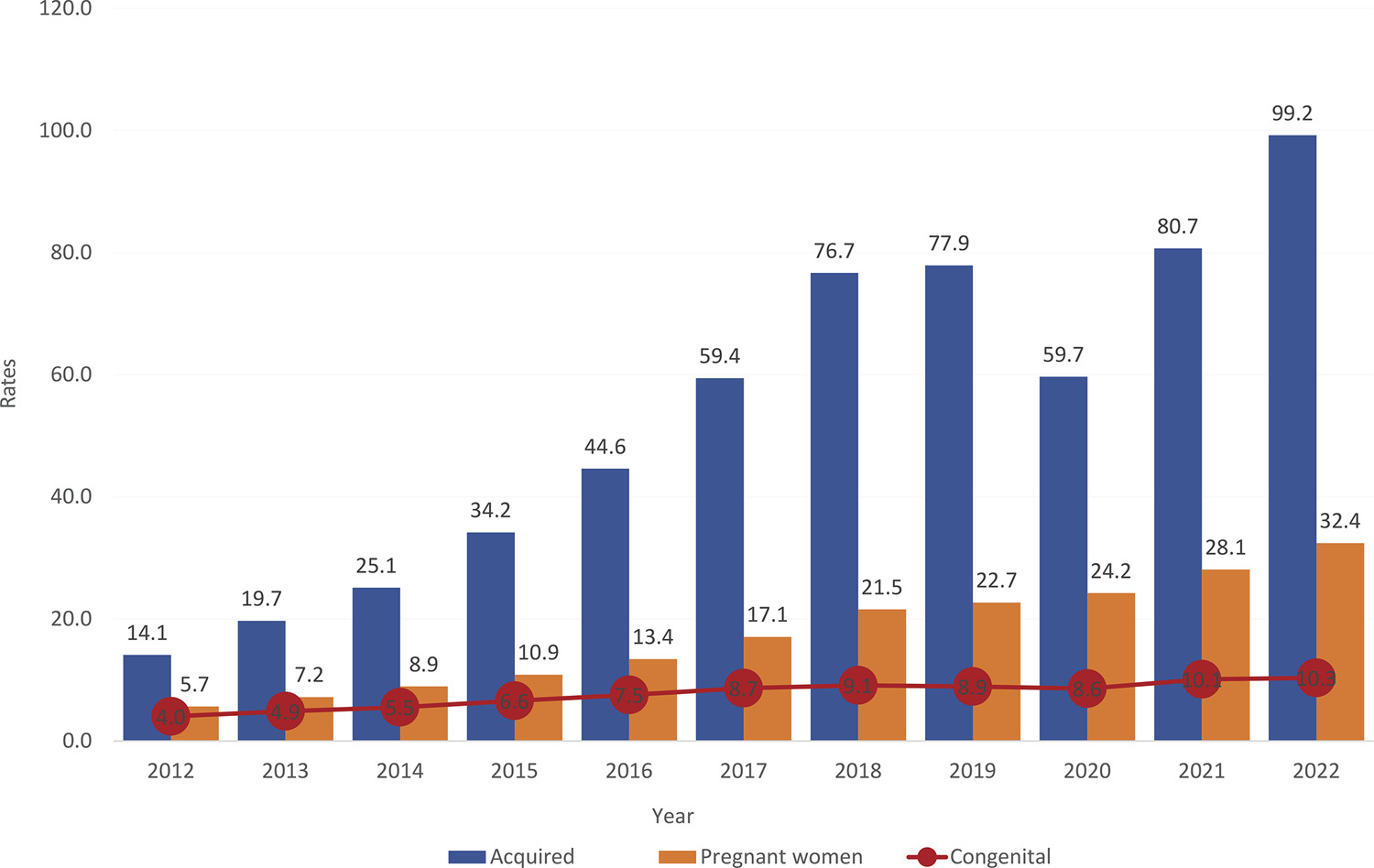
•Although congenital syphilis has a known etiological agent, accessible diagnosis and low-cost, effective treatment with low fetal toxicity, it continues to challenge obstetric and antenatal care services.
•The increasing rates of syphilis in the general population have direct repercussions on the increase in cases of congenital syphilis, a situation of objective interest for public health.
•Although transforming the recording of syphilis and congenital syphilis into notifiable diseases improved the records and has made it possible to measure the occurrence of these diseases and create solutions, no effects on reducing their frequency have been reached yet.
•The failure to control syphilis/congenital syphilis is multifactorial, and associates variables that range from the deficiency in teaching about these diseases in schools and in the training system of the various health professional segments, as well as the lack of rigid policies for quality control from antenatal care until the clinical follow-up of children exposed to Treponema pallidum during pregnancy.
•To date, benzathine penicillin is the only antimicrobial accepted as effective by the main health authorities on the planet for the treatment of syphilis in pregnant women.
•The fear of anaphylaxis in response to the treatment of syphilis with benzathine penicillin is an important factor hindering the prompt and correct treatment of pregnant women with syphilis, even though health authorities have made efforts to face the problem with solid arguments, still insufficient to resolve the question.
•Although specific protocols are published, the failure to control the treatment of syphilis in pregnant women is still observed with high frequency, indicating and reinforcing a failure in the quality control of these care principles.
The National Specialized Commission on Infectious Diseases of the Brazilian Federation of Gynecology and Obstetrics Associations (Febrasgo) endorses this document. Content production is based on scientific evidence on the proposed topic and the results presented contribute to clinical practice.
-
Review Article09-18-2024
Immunosuppressants in women with repeated implantation failure in assisted reproductive techniques: a systematic review and meta-analysis
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo70
Abstract
Review ArticleImmunosuppressants in women with repeated implantation failure in assisted reproductive techniques: a systematic review and meta-analysis
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo70
Views248Abstract
Objective
To compare outcomes in patients with repeated implantation failure undergoing Intracytoplasmic Sperm Injection/In vitro fertilization (IVF/ICSI) plus immunosuppressants such as prednisolone, prednisone, or cyclosporine A versus the use of IVF/ICSI alone.
Data source
Databases were systematically searched in PubMed, Cochrane, and Embase databases in September 2023.
Study Selection
Randomized clinical trials and observational studies with the outcomes of interest were included.
Data collect
We computed odds ratios (ORs) for binary endpoints, with 95% confidence intervals (CIs). Heterogeneity was assessed using I2 statistics. Data were analyzed using Review Manager 5.4.The main outcomes were live birth, miscarriage, implantation rate, clinical pregnancy, and biochemical pregnancy.
Data synthesis
Seven studies with 2,829 patients were included. Immunosuppressive treatments were used in 1,312 (46.37%). Cyclosporine A improved implantation rate (OR 1.48; 95% CI 1.01-2.18) and clinical pregnancy (1.89, 95% CI 1.14-3.14). Compared to non-immunosuppressive treatment, prednisolone and prednisone did not improve live birth (OR 1.13, 95% CI 0.88-1.46) and miscarriage (OR 1.49, 95% CI 1.07-2.09). Prednisolone showed no significant effect in patients undergoing IVF/ICSI, clinical pregnancy (OR 1.34; 95% CI 0.76-2.36), or implantation rate (OR 1.36; 95% CI 0.76-2.42).
Conclusion
Cyclosporine A may promote implantation and clinical pregnancy rates. However, given the limited sample size, it is important to approach these findings with caution. Our results indicate that prednisolone and prednisone do not have any beneficial effects on clinical outcomes of IVF/ICSI patients with repeated implantation failure.
PROSPERO
CRD42023449655
Key-words Cyclosporine APrednisolone Immunosupressive agentsPrednisoneRepeated implantation failureReproductionReproductive techniques, assistedSee more
Search
Search in:
Tag Cloud
Pregnancy (252)Breast neoplasms (104)Pregnancy complications (104)Risk factors (103)Menopause (88)Ultrasonography (83)Cesarean section (78)Prenatal care (71)Endometriosis (70)Obesity (61)Infertility (57)Quality of life (55)prenatal diagnosis (51)Women's health (48)Postpartum period (46)Maternal mortality (45)Pregnant women (45)Breast (44)Prevalence (43)Uterine cervical neoplasms (43)