Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS10
•The negative impact of infectious diseases and their immunoprevention during the different stages of a woman’s life requires a broad approach including adolescence, adulthood, pregnancy and the postmenopausal phase.
•Immunization of pregnant women should be a priority for the protection of the maternal-fetal dyad, especially in regions with high rates of infections preventable by immunization.
•Brazil has one of the most comprehensive vaccination programs in the world – the National Immunization Program (Programa Nacional de Imunizações, PNI) – that serves all age groups: newborns, children, adolescents, adults, pregnant women and older adults, as well as groups with special needs, such as adolescents, pregnant and older adult women.
•However, vaccination coverage remains below ideal for all available vaccines, especially among adolescents and pregnant women, and Febrasgo is committed to collaborating with the PNI to combat vaccine hesitancy.
•The gynecologist/obstetrician is the reference physician for women, therefore the access to information and updates regarding all vaccines recommended for their patients is extremely important for this professional, aiming at the greatest possible protection.
•The objective of this Febrasgo Position Statement is to bring an update to women’s vaccination schedule, covering some vaccines that are available, including new approved vaccines and those in the commercialization phase.
•This work is a compilation of the First Febrasgo Scientific Immunization Forum held in the city of São Paulo in October 2023 with the objective to update recommendations for vaccines in use and new innovative vaccines soon to be available.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo100
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS09
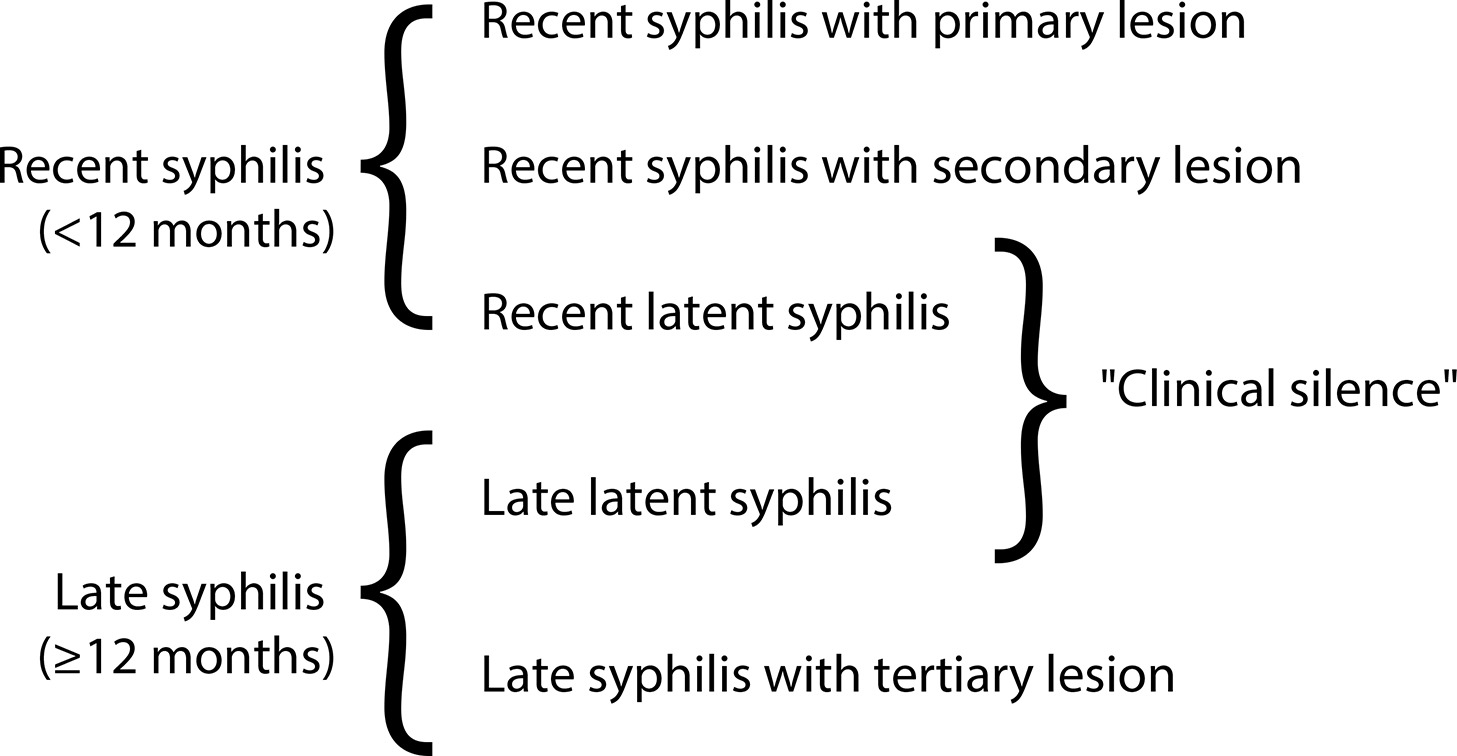
•Although congenital syphilis has a known etiological agent, accessible diagnosis and low-cost, effective treatment with low fetal toxicity, it continues to challenge obstetric and antenatal care services.
•The increasing rates of syphilis in the general population have direct repercussions on the increase in cases of congenital syphilis, a situation of objective interest for public health.
•Although transforming the recording of syphilis and congenital syphilis into notifiable diseases improved the records and has made it possible to measure the occurrence of these diseases and create solutions, no effects on reducing their frequency have been reached yet.
•The failure to control syphilis/congenital syphilis is multifactorial, and associates variables that range from the deficiency in teaching about these diseases in schools and in the training system of the various health professional segments, as well as the lack of rigid policies for quality control from antenatal care until the clinical follow-up of children exposed to Treponema pallidum during pregnancy.
•To date, benzathine penicillin is the only antimicrobial accepted as effective by the main health authorities on the planet for the treatment of syphilis in pregnant women.
•The fear of anaphylaxis in response to the treatment of syphilis with benzathine penicillin is an important factor hindering the prompt and correct treatment of pregnant women with syphilis, even though health authorities have made efforts to face the problem with solid arguments, still insufficient to resolve the question.
•Although specific protocols are published, the failure to control the treatment of syphilis in pregnant women is still observed with high frequency, indicating and reinforcing a failure in the quality control of these care principles.
The National Specialized Commission on Infectious Diseases of the Brazilian Federation of Gynecology and Obstetrics Associations (Febrasgo) endorses this document. Content production is based on scientific evidence on the proposed topic and the results presented contribute to clinical practice.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo69
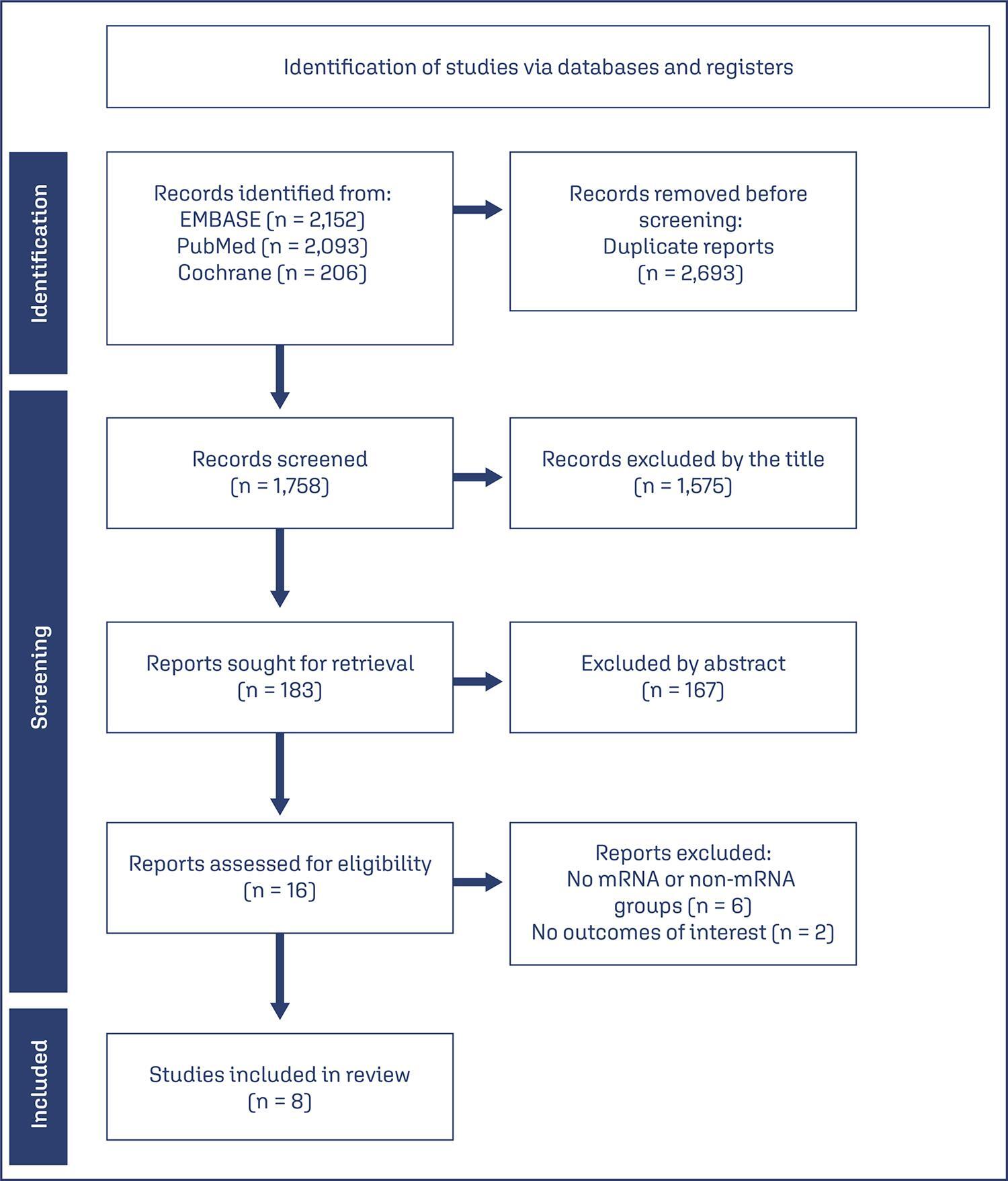
To compare the effectiveness and safety of non-mRNA versus mRNA COVID-19 vaccines on pregnant women and their newborns in a systematic review with meta-analysis.
We searched PubMed, Embase, and Cochrane Central in May 2023.
The search strategy yielded 4451 results, 16 studies were fully reviewed. We selected case-control studies analysing non-mRNA versus mRNA vaccines. Data collection and analysis: we assessed the risk of bias using the Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool. Standardised mean differences were pooled using random-effect models.
We identified 8 prospective and retrospective studies with a total of 32,153 patients. Non-mRNA vaccines were associated with a higher incidence of fever (OR 2.67; 95% CI 2.08-3.43; p<0.001), and a lower incidence of fetal or neonatal death (OR 0.16; 95% CI 0.08-0.33; p<0.001). In subgroup analyses, the Jansen vaccine (Ad26.COV2.S) was found to have a higher rate of premature labor/delivery (OR 4.48; 95% CI 1.45-13.83; p=0.009) and missed/spontaneous abortion (OR 1.90; 95% CI 1.09-3.30; p=0.02), as compared with the Pfizer (BNT162b2) vaccine.
non-mRNA vaccines are associated with a lower incidence of fetal or neonatal death among pregnant women who receive a Covid19 vaccine, although at an increased rate of pyrexia compared with mRNA vaccines. Other studies are required for better assessment.
CRD42023421814
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo67
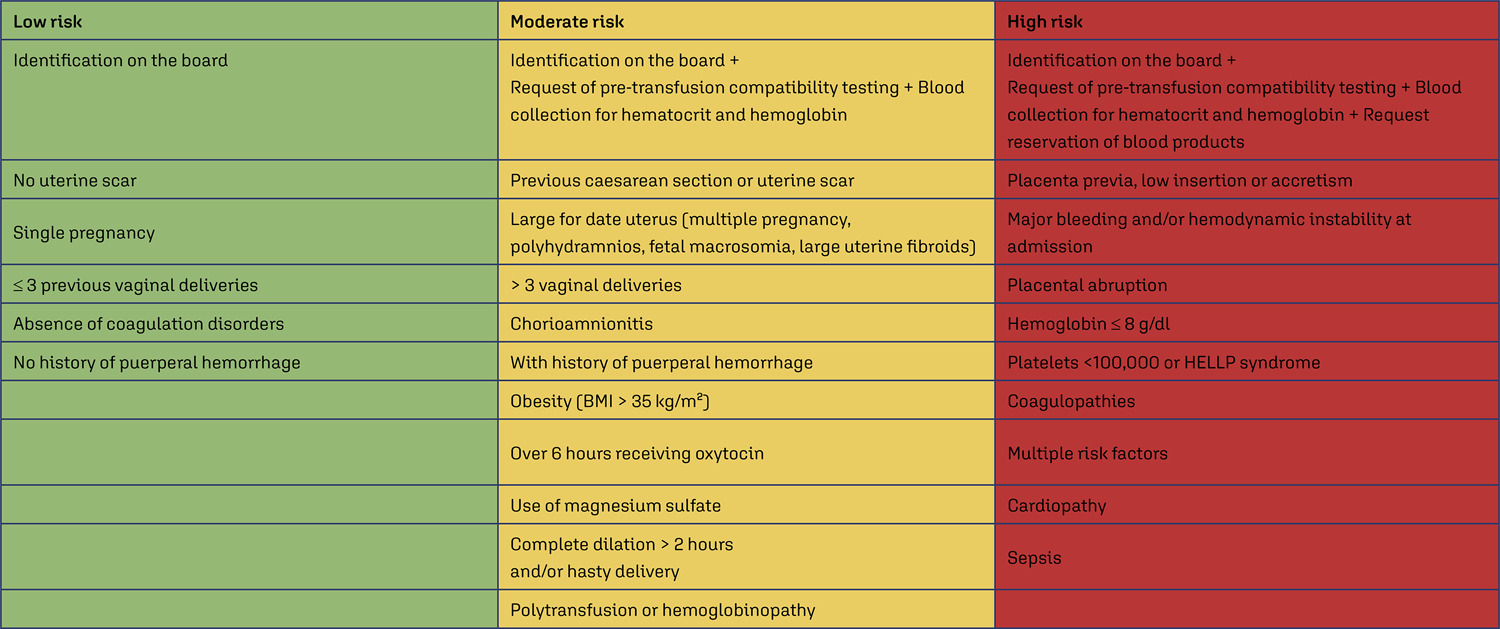
Compare the number of puerperal women submitted to blood transfusion before and after the implementation of a care protocol for postpartum hemorrhage (PPH) with multidisciplinary team training.
Cross-sectional study in a university hospital, analyzing births from 2015 to 2019, compared the use of blood products before and after the adoption of a PPH protocol with multidisciplinary training.
Between 2015 and 2019, there were 17,731 births, with 299 (1.7%) postpartum women receiving blood products and 278 postpartum women were considered for this analysis, 128 (0.7%) at Time 1 and 150 (0.8%) at Time 2. After the multiprofessional team training (T2), there was a difference in the complete use of the PPH protocol (use of oxytocin, misoprostol and tranexamic acid) (T1 = 5.1% x T2 = 49.5%, p≤0.0001). An individual categorized analysis revealed that, in the T2 period, there was lower use of blood component units per patient compared to T1 (Mann-Whitney, p=0.006). It should be noted that at T1 and T2, 54% and 24% respectively received two units of blood products. It is important to highlight that after the multidisciplinary team training for the PPH protocol, the goal of zero maternal death due to hemorrhage was reached.
The adoption of a specific protocol for PPH, combined with the training of a multidisciplinary team, had an impact on the ability to identify women at high risk of hemorrhage, resulting in a decrease in the use of blood components.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo70
To compare outcomes in patients with repeated implantation failure undergoing Intracytoplasmic Sperm Injection/In vitro fertilization (IVF/ICSI) plus immunosuppressants such as prednisolone, prednisone, or cyclosporine A versus the use of IVF/ICSI alone.
Databases were systematically searched in PubMed, Cochrane, and Embase databases in September 2023.
Randomized clinical trials and observational studies with the outcomes of interest were included.
We computed odds ratios (ORs) for binary endpoints, with 95% confidence intervals (CIs). Heterogeneity was assessed using I2 statistics. Data were analyzed using Review Manager 5.4.The main outcomes were live birth, miscarriage, implantation rate, clinical pregnancy, and biochemical pregnancy.
Seven studies with 2,829 patients were included. Immunosuppressive treatments were used in 1,312 (46.37%). Cyclosporine A improved implantation rate (OR 1.48; 95% CI 1.01-2.18) and clinical pregnancy (1.89, 95% CI 1.14-3.14). Compared to non-immunosuppressive treatment, prednisolone and prednisone did not improve live birth (OR 1.13, 95% CI 0.88-1.46) and miscarriage (OR 1.49, 95% CI 1.07-2.09). Prednisolone showed no significant effect in patients undergoing IVF/ICSI, clinical pregnancy (OR 1.34; 95% CI 0.76-2.36), or implantation rate (OR 1.36; 95% CI 0.76-2.42).
Cyclosporine A may promote implantation and clinical pregnancy rates. However, given the limited sample size, it is important to approach these findings with caution. Our results indicate that prednisolone and prednisone do not have any beneficial effects on clinical outcomes of IVF/ICSI patients with repeated implantation failure.
CRD42023449655
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgoedt4
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo39i
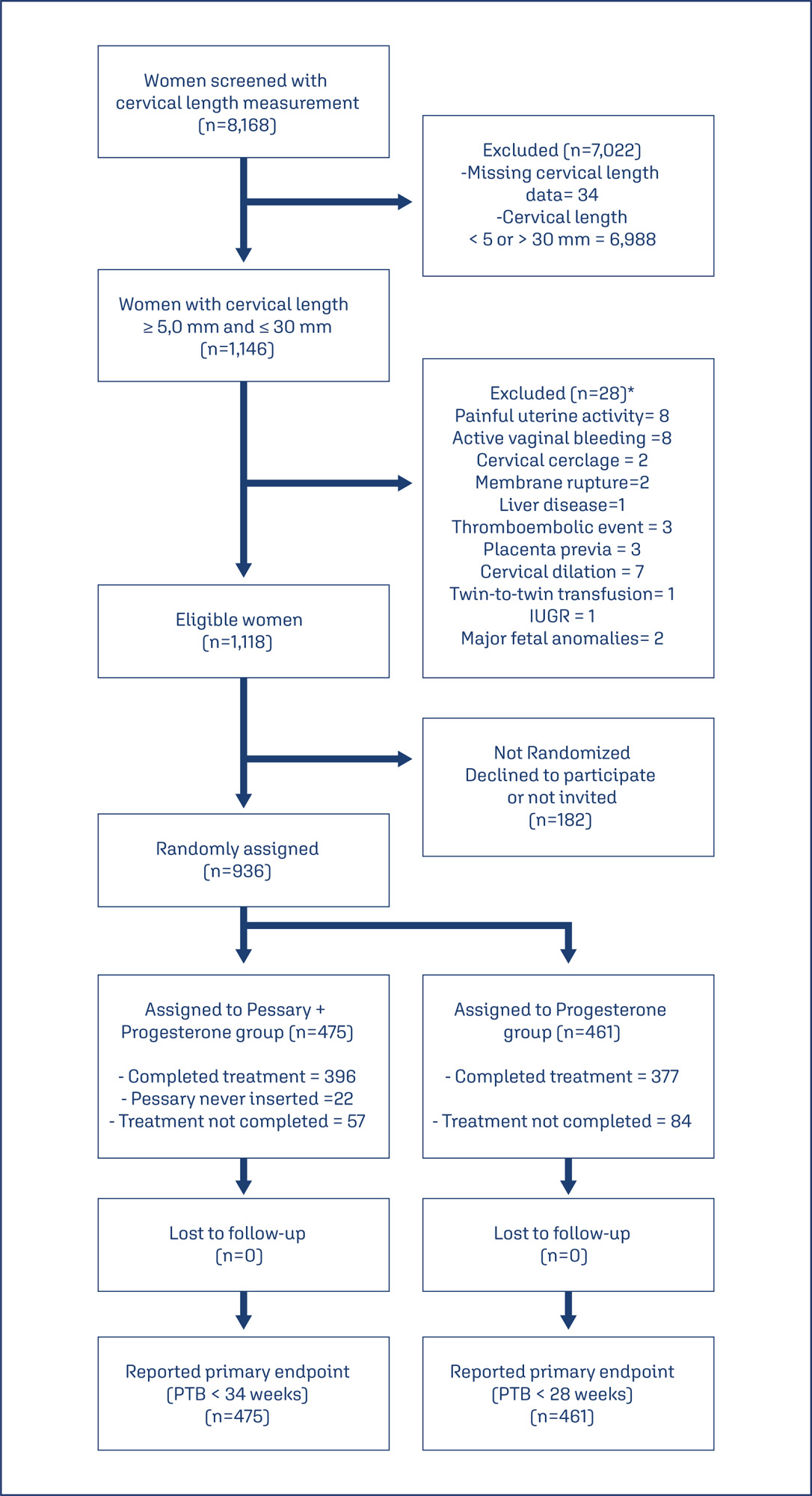
This study aims to create a new screening for preterm birth < 34 weeks after gestation with a cervical length (CL) ≤ 30 mm, based on clinical, demographic, and sonographic characteristics.
This is a post hoc analysis of a randomized clinical trial (RCT), which included pregnancies, in middle-gestation, screened with transvaginal ultrasound. After observing inclusion criteria, the patient was invited to compare pessary plus progesterone (PP) versus progesterone only (P) (1:1). The objective was to determine which variables were associated with severe preterm birth using logistic regression (LR). The area under the curve (AUC), sensitivity, specificity, and positive predictive value (PPV) and negative predictive value (NPV) were calculated for both groups after applying LR, with a false positive rate (FPR) set at 10%.
The RCT included 936 patients, 475 in PP and 461 in P. The LR selected: ethnics white, absence of previous curettage, previous preterm birth, singleton gestation, precocious identification of short cervix, CL < 14.7 mm, CL in curve > 21.0 mm. The AUC (CI95%), sensitivity, specificity, PPV, and PNV, with 10% of FPR, were respectively 0.978 (0.961-0.995), 83.4%, 98.1%, 83.4% and 98.1% for PP < 34 weeks; and 0.765 (0.665-0.864), 38.7%, 92.1%, 26.1% and 95.4%, for P < 28 weeks.
Logistic regression can be effective to screen preterm birth < 34 weeks in patients in the PP Group and all pregnancies with CL ≤ 30 mm.