Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(5):275-280
Gender incongruence is defined as a condition in which an individual self-identifies and desires to have physical characteristics and social roles that connote the opposite biological sex. Gender dysphoria is when an individual displays the anxiety and/or depression disorders that result from the incongruity between the gender identity and the biological sex. The gender affirmation process must be performed by a multidisciplinary team. The main goal of the hormone treatment is to start the development of male physical characteristics by means of testosterone administration that may be offered to transgender men who are 18 years old or over. The use of testosterone is usually well tolerated and improves the quality of life. However, there is still lack of evidence regarding the effects and risks of the long-term use of this hormone. Many different pharmacological formulations have been used in the transsexualization process. The most commonly used formulation is the intramuscular testosterone esters in a short-term release injection, followed by testosterone cypionate or testosterone enanthate. In the majority of testosterone therapy protocols, the male physical characteristics can be seen in almost all users after 6 months of therapy, and themaximum virilization effects are usually achieved after 3 to 5 years of regular use of the hormone. To minimize risks, plasmatic testosterone levels should be kept within male physiological ranges (300 to 1,000 ng/dl) during hormonal treatment. It is recommended that transgender men under androgen therapy be monitored every 3 months during the 1st year of treatment and then, every 6 to 12 months.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(5):287-293
To perform a comprehensive review of the current evidence on the role of uterine artery Doppler, isolated or in combination with other markers, in screening for preeclampsia (PE) and fetal growth restriction (FGR) in the general population. The review included recently published large cohort studies and randomized trials.
A search of the literature was conducted usingMedline, PubMed, MeSH and ScienceDirect. Combinations of the search terms “preeclampsia,” “screening,” “prediction,” “Doppler,” “Doppler velocimetry,” “fetal growth restriction,” “small for gestational age” and “uterine artery” were used. Articles in English (excluding reviews) reporting the use of uterine artery Doppler in screening for PE and FGR were included.
Thirty articles were included. As a single predictor, uterine artery Doppler detects less than 50% of the cases of PE and no more than 40% of the pregnancies affected by FGR. Logistic regression-based models that allow calculation of individual risk based on the combination of multiple markers, in turn, is able to detect ~ 75% of the cases of preterm PE and 55% of the pregnancies resulting in small for gestational age infants.
The use of uterine artery Doppler as a single predictive test for PE and FGR has poor accuracy. However, its combined use in predictive models is promising, being more accurate in detecting preterm PE than FGR.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(4):209-224
To review the existing recommendations on the prenatal care of women with systemic lupus erythematosus (SLE), based on currently available scientific evidence.
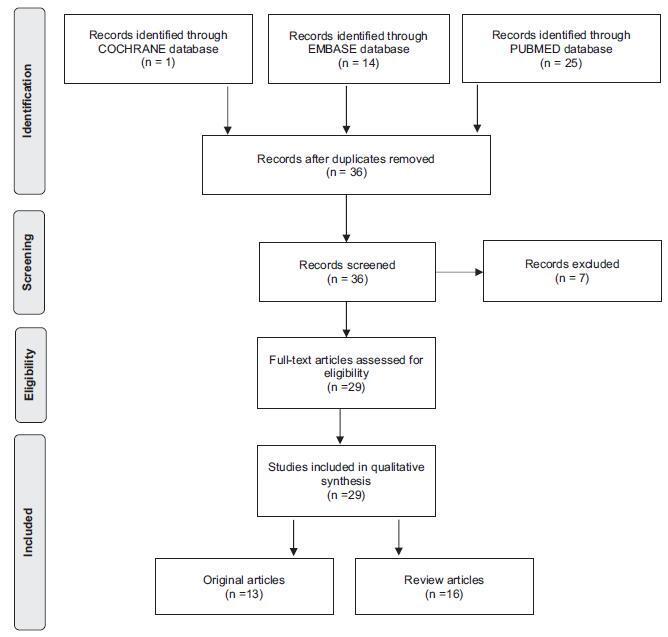
An integrative review was performed by two independent researchers, based on the literature available in the MEDLINE (via PubMed), EMBASE and The Cochrane Library databases, using the medical subject headings (MeSH) terms “systemic lupus erythematosus” AND “high-risk pregnancy” OR “prenatal care.” Studies published in English between 2007 and 2017 were included; experimental studies and case reports were excluded. In cases of disagreement regarding the inclusion of studies, a third senior researcher was consulted. Forty titles were initially identified; four duplicates were excluded. After reading the abstracts, 7 were further excluded and 29 were selected for a full-text evaluation.
Systemic lupus erythematosus flares, preeclampsia, gestation loss, preterm birth, fetal growth restriction and neonatal lupus syndromes (mainly congenital heartblock) were the major complications described. The multidisciplinary team should adopt a specific monitoring, with particular therapeutic protocols. There are safe and effective drug options that should be prescribed for a good control of SLE activity.
Pregnant women with SLE present an increased risk for maternal complications, pregnancy loss and other adverse outcomes. The disease activity may worsen and, thereby, increase the risk of other maternal-fetal complications. Thus, maintaining an adequate control of disease activity and treating flares quickly should be a central goal during prenatal care.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(4):225-231
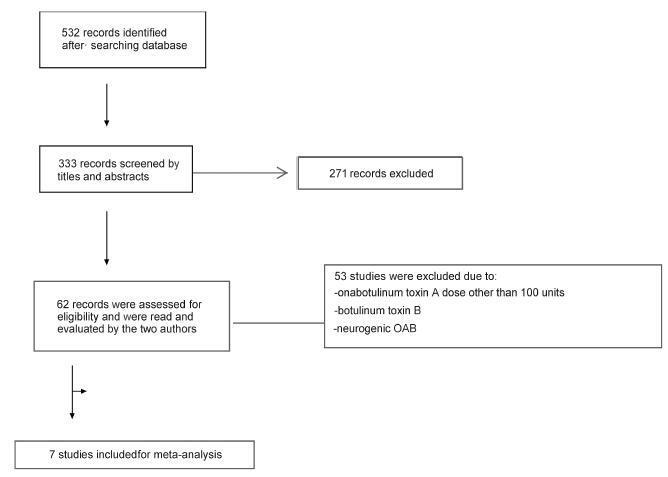
We performed a systematic review and meta-analysis of randomized placebo-controlled trials that studied non-neurogenic overactive bladder patients who were treated with 100 units of onabotulinumtoxinA or placebo. The primary purpose of our study was to evaluate the clinical effectiveness with regard to urinary urgency, urinary frequency, nocturia, and incontinence episodes. Our secondary purpose consisted of evaluating the adverse effects. Our initial search yielded 532 entries. Of these, seven studies met all the inclusion criteria (prospective, randomized, placebo-controlled studies, ≥ 3 points on the Jadad scale) and were selected for analysis. For all primary endpoints, the toxin was more effective than placebo (p < 0.0001; 95% confidence interval [95CI]), namely: urgency (mean difference = -2.07; 95CI = [-2.55-1.58]), voiding frequency (mean difference = - 1.64; 95CI = [-2.10-1.18]), nocturia (mean difference = -0.25; 95CI = [-0.39-0.11]) and incontinence episodes (mean difference = -2.06; 95CI= [-2.60-1.52]). The need for intermittent catheterization and the occurrence of urinary tract infection (UTI) were more frequent in patients treated with onabotulinumtoxinA than in patients treated with placebo (p < 0.0001). Compared with placebo, onabotulinumtoxinA had significantly and clinically relevant reductions in overactive bladder symptoms and is associated with higher incidence of intermittent catheterization and UTI.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(3):147-155
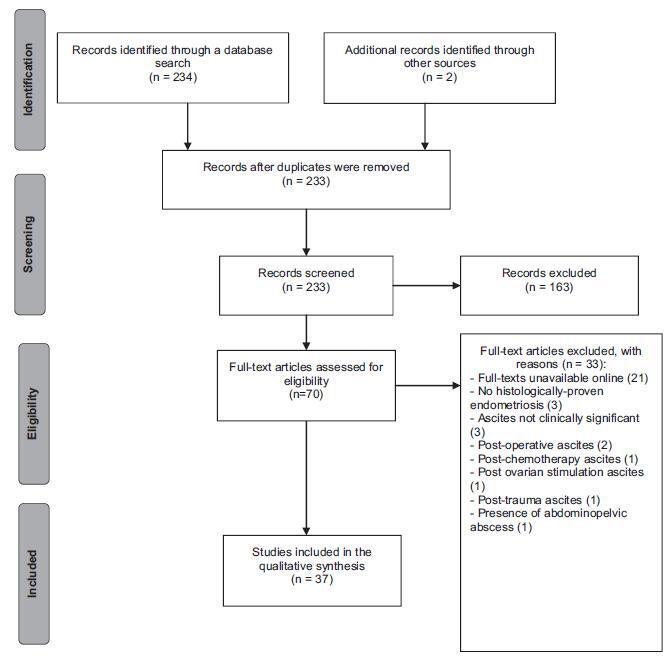
Endometriosis can have several different presentations, including overt ascites and peritonitis; increased awareness can improve diagnostic accuracy and patient outcomes. We aimto provide a systematic review and report a case of endometriosis with this unusual clinical presentation. The PubMed/MEDLINE database was systematically reviewed until October 2016. Women with histologically-proven endometriosis presenting with clinically significant ascites and/or frozen abdomen and/or encapsulating peritonitis were included; thosewith potentially confounding conditionswere excluded.Our search yielded 37 articles describing 42 women, all of reproductive age. Ascites was mostly hemorrhagic, recurrent and not predicted by cancer antigen 125 (CA-125) levels. In turn, dysmenorrhea, dyspareunia and infertility were not consistently reported. The treatment choices and outcomes were different across the studies, and are described in detail. Endometriosis should be a differential diagnosis of massive hemorrhagic ascites in women of reproductive age.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(3):156-162
Venous thromboembolism events are important causes of maternal death during pregnancyandthepostpartumperiodworldwide.Are view of the literature with the objective of evaluating venous thromboembolism events in the puerperium according to the route of delivery was performed through a bibliographic survey in the Medline, LILACS and Scielo databases. We observed that patients submitted to cesarean sections present a significantlyhigher riskofdeveloping venousthromboembolismwhencomparedwiththose who undergo spontaneous vaginal delivery. The pathophysiological bases for this difference were explored and described in this review, as well as the indications of prophylaxis and treatment. Doctors and health professionals must be continuously vigilant regarding this condition, since it is associated with high morbidity and mortality.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(2):96-102
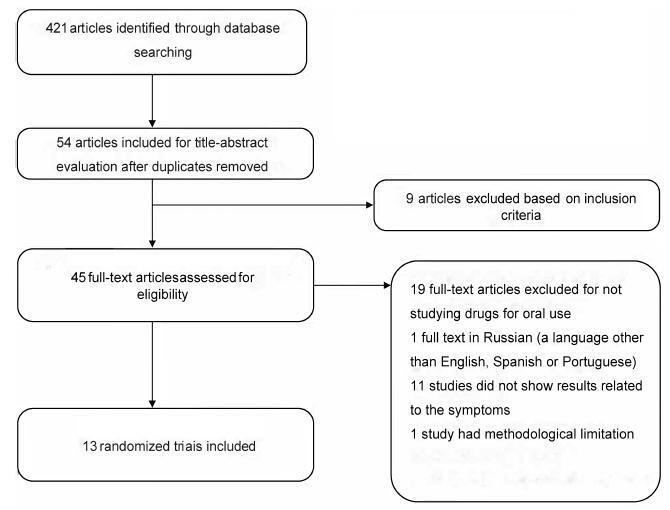
Interstitial cystitis (IC), including bladder pain syndrome (BPS), is a chronic and debilitating disease thatmainly affectswomen. It is characterized by pelvic pain associated with urinary urgency, frequency, nocturia and negative urine culture,with normal cytology. In 2009, the Society for Urodynamics and Female Urology (SUFU) defined the term IC/BPS as an unpleasant sensation (pain, pressure, and discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms for more than 6 weeks duration, in the absence of infection or other identifiable causes. This is the definition used by the American Urological Association (AUA) in the most recent guidelines on IC/BPS. Interstitial cystitis may be sufficiently severe to have a devastating effect on the quality of life, but it may also be associated with moderate symptoms whose effects are less debilitating. Although there are several clinical trials to assess oral and intravesical therapies, the treatment for IC remains far from ideal. This systematic assessment evaluates published randomized clinical trials on oralmedications used totreat symptoms of BPS. This studywas performed according to the preferred reporting items for systematic reviews and metaanalyses (PRISMA)method. Two independent reviewers screened the studies to determine their inclusion or exclusion and to perform the methodological analysis. The inclusion criteria included randomized studiespublishedbetween April of 1988and April of2016 that used oral medications to treat symptoms of BPS or IC. According to the systematic review performed,we should consider pentosan polysulfate as one of the bestoptions of oral drugs for the treatment of BPS symptoms. However, this drug is not an available option in Brazil. Orally administered amitriptyline is an efficacious medical treatment for BPS, and it should be the first treatment offered.