-
Original Article
Access and adequacy of antenatal care in a city in Brazil during two phases of the COVID-19 pandemic
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo87
10-23-2024
Summary
Original ArticleAccess and adequacy of antenatal care in a city in Brazil during two phases of the COVID-19 pandemic
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo87
10-23-2024Views148Abstract
Objective:
To compare access and suitability of antenatal care between years 2020 and 2022 among postpartum individuals at a Hospital in Florianopolis, and evaluate factors associated with antenatal suitability.
Methods:
Observational, cross-sectional, and quantitative study carried out in 2022. Collected data were compared with the database of a previous similar study carried out in the same setting in 2020. Data were extracted from medical records and prenatal booklets, in addition to a face-to-face questionnaire. Adequacy was measured using the Carvalho and Novaes index and health access was qualitatively evaluated. Socio-demographic and antenatal variables were analyzed. A statistical significance level of 0.05 was considered. Open-ended questions were categorized for analysis.
Results:
395 postpartum individuals were included. Antenatal care was adequate for 48.6% in 2020 and 69.1% in 2022. Among the barriers to access, 56% reported difficulty in scheduling appointments and/or exams and 23% complained of reduced healthcare staff due to strikes, COVID-19, among others. Adequate antenatal care was associated with being pregnant in 2022, being referred to high-risk units (PNAR), and not reporting difficulties in access. Also, it was associated with twice the chance of investigation for gestational diabetes (GDM) and syphilis.
Conclusion:
The 2022 post-vaccination period showed higher antenatal adequacy. The main difficulty for postpartum individuals was scheduling appointments and/or exams. Having antenatal care in 2022, no reports of difficulty in access, and follow-up at a high-risk unit were associated with antenatal adequacy.
Key-words COVID-19Delivery of health careDiabetesGestationalpandemicsPostpartum periodPregnancyPrenatal caresurveys and questionnairesVaccinationSee more -
Review Article
Technologies Applied to the Mental Health Care of Pregnant Women: A Systematic Literature Review
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(3):149-159
07-10-2023
Summary
Review ArticleTechnologies Applied to the Mental Health Care of Pregnant Women: A Systematic Literature Review
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(3):149-159
07-10-2023Views189See moreAbstract
Objective:
This article aims to review the literature regarding the use of technologies to promote mental health for pregnant women. We seek to: understand the strategies that pregnant women use for mental health care. Also, we investigate the existence of scientific evidence that validates such practices.
Methods:
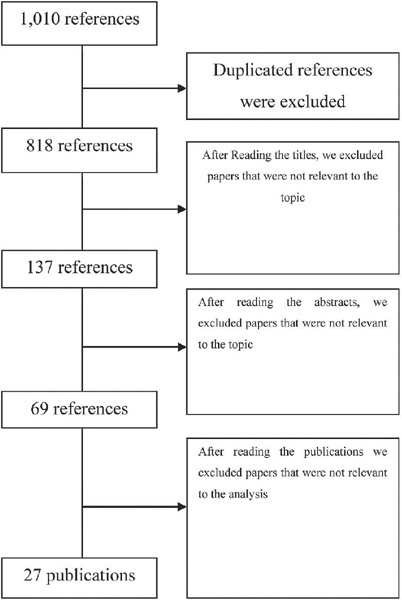
This study follows the PRISMA guidelines for systematic reviews. We analyze 27 studies published between 2012 and 2019. We include publications in Portuguese, English, and Spanish.
Results:
The results revealed several different possibilities to use technology, including the use of text messages and mobile applications on smartphones. Mobile applications are the most commonly used approaches (22.5%). Regarding the strategies used, cognitive-behavioral approaches, including mood checks, relaxation exercises, and psychoeducation comprised 44.12% of the content.
Conclusion:
There is a need for further investigation and research and development efforts in this field to better understand the possibilities of intervention in mental health in the digital age.
-
Original Article
Development of a Mobile Health Application Based on a Mixed Prenatal Care in the Context of COVID-19 Pandemic
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(4):179-185
06-30-2023
Summary
Original ArticleDevelopment of a Mobile Health Application Based on a Mixed Prenatal Care in the Context of COVID-19 Pandemic
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(4):179-185
06-30-2023Views132See moreAbstract
Objective
We describe the development and structure of a novel mobile application in a mixed model of prenatal care, in the context of the COVID-19 pandemic. Furthermore, we assess the acceptability of this mobile app in a cohort of patients.
Methods
First, we introduced a mixed model of prenatal care; second, we developed a comprehensive, computer-based clinical record to support our system. Lastly, we built a novel mobile app as a tool for prenatal care. We used Flutter Software version 2.2 to build the app for Android and iOS smartphones. A cross-sectional study was carried out to assess the acceptability of the app.
Results
A mobile app was also built with the main attribute of being connected in real-time with the computer-based clinical records. The app screens detail information about activities programmed and developed in the prenatal care according to gestational age. A downloadable maternity book is available and some screens show warning signs and symptoms of pregnancy. The acceptability assessment was mostly rated positively regarding the characteristics of the mobile app, by 50 patients.
Conclusion
This novel mobile app was developed as a tool among pregnant patients to increase the information available about their pregnancies in the provision of a mixed model of prenatal care in the context of the COVID-19 pandemic. It was fully customized to the needs of our users following the local protocols. The introduction of this novel mobile app was highly accepted by the patients.
-
Original Article
Low-Risk Antenatal Care Enhanced by Telemedicine: A Practical Guideline Model
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(9):845-853
07-19-2022
Summary
Original ArticleLow-Risk Antenatal Care Enhanced by Telemedicine: A Practical Guideline Model
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(9):845-853
07-19-2022Views167See moreAbstract
Objective
To develop a protocol for hybrid low-risk prenatal care adapted to Brazilian guidelines, merging reduced face-to-face consultations and remote monitoring.
Methods
The PubMed, Embase, and Cochrane Library databases were systematically searched on telemedicine and antenatal care perspectives and adaptation of the low-risk prenatal care protocols recommended by the Ministry of Health and by the Brazilian Federation of Gynecology and Obstetrics Associations.
Results
Five relevant articles and three manuals were included in the review, for presented criteria to develop this clinical guideline. We identified, in these studies, that the schedule of consultations is unevenly distributed among the gestational trimesters, and ranges from 7 to 14 appointments. In general, the authors propose one to two appointments in the first trimester, two to three appointments in the second trimester, and two to six appointments in the third trimester. Only three studies included puerperal evaluations. The routine exams recommended show minimal variations among authors. To date, there are no validated Brazilian protocols for prenatal care by telemedicine. The included studies showed that pregnant women were satisfied with this form of care, and the outcomes of interest, except for hypertensive diseases, were similar between the groups exposed to traditional and hybrid prenatal care.
Conclusion
The presented guideline comprises the Ministry of Health recommendations for low-risk prenatal care and reduces exposure to the hospital environment and care costs. A randomized clinical trial, to be developed by this group, will provide real-world data on safety, effectiveness, satisfaction, and costs.
-
Original Article
Screening of Perinatal Depression Using the Edinburgh Postpartum Depression Scale
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(5):452-457
03-04-2022
Summary
Original ArticleScreening of Perinatal Depression Using the Edinburgh Postpartum Depression Scale
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(5):452-457
03-04-2022Views304See moreAbstract
Objective
To detect depression during pregnancy and in the immediate postpartum period using the Edinburgh postpartum depression scale (EPDS).
Methods
Cross sectional study of 315 women, aged between 14 and 44 years, who received perinatal care at the Leonor Mendes de Barros Hospital, in São Paulo, between July 1st, 2019 and October 30th, 2020. The cutoff point suggesting depression was ≥ 12.
Results
The screening indicated 62 (19.7%) patients experiencing depression. Low family income, multiparity, fewer prenatal appointments, antecedents of emotional disorders, dissatisfaction with the pregnancy, poor relationship with the partner, and psychological aggression were all risk factors associated with depression in pregnancy or in the immediate postpartum period. Antecedents of depression and psychology aggression during pregnancy were significant variables for predicting perinatal depression in the multivariate analysis.
Conclusion
There is a significant association between the occurrence of perinatal depression and the aforementioned psychosocial factors. Screening patients with the EPDS during perinatal and postpartum care could facilitate establishing a line of care to improve the wellbeing of mother and infant.
-
Original Article
Adequacy of Antenatal Care during the COVID-19 Pandemic: Observational Study with Postpartum Women
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(4):398-408
02-17-2022
Summary
Original ArticleAdequacy of Antenatal Care during the COVID-19 Pandemic: Observational Study with Postpartum Women
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(4):398-408
02-17-2022Views39See moreAbstract
Objective
The present study aimed to evaluate the antenatal care adequacy for women who gave birth at the University Hospital of Santa Catarina in Florianopolis (Brazil) during the COVID-19 pandemic, and to evaluate the association of adequacy with sociodemographic, clinical, and access characteristics.
Methods
Data were collected between October and December 2020, including 254 patients who delivered in the University Hospital from Federal University of Santa Catarina and answered our questionnaires. Additional data were obtained from patients’ antenatal booklets. Antenatal care was classified as adequate, intermediate, or inadequate according to the number of appointments, gestational age at the beginning of follow-up, and tests results. We carried out a descriptive statistical analysis and a bivariate/with odds ratio analysis onmaternal sociodemographic, clinical and health access variables that were compared with antenatal adequacy.
Results
Antenatal care was considered adequate in 35.8% of cases, intermediate in 46.8%, and inadequate in 17.4%. The followingmaternal variables were associated with inadequate prenatal care (intermediate or inadequate prenatal care): having black or brown skin colour, having two or more children, being of foreign nationality, not being fluent in Portuguese, and using illicit drugs during pregnancy; the clinical variables were more than 6 weeks between appointments, and not attending high-risk antenatal care; as for access, the variables were difficulties in attending or scheduling appointments, and attending virtual appointments only.
Conclusion
In a sample of pregnant women from a teaching hospital in Florianópolis during the COVID-19 pandemic, antenatal care was considered adequate in 35.8%, intermediate in 46.8%, and inadequate in 17.4% of cases.
-
Original Article
Impact of Carbohydrate Counting Method during Pregnancy in Women with Pregestational Diabetes Mellitus: A Controlled Clinical Trial
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(3):220-230
02-09-2022
Summary
Original ArticleImpact of Carbohydrate Counting Method during Pregnancy in Women with Pregestational Diabetes Mellitus: A Controlled Clinical Trial
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(3):220-230
02-09-2022Views162See moreAbstract
Objective
To evaluate the effect of the carbohydrate counting method (CCM) on glycemic control,maternal, and perinatal outcomes of pregnant women with pregestational diabetes mellitus (DM).
Methods
Nonrandomized controlled clinical trial performed with 89 pregnant women who had pregestational DMand received prenatal care in a public hospital in Rio de Janeiro, state of Rio de Janeiro, Brazil, between 2009 and 2014, subdivided into historic control group and intervention group, not simultaneous. The intervention group (n=51) received nutritional guidance from the carbohydrate counting method (CCM), and the historical control group (n=38), was guided by the traditionalmethod (TM). The Mann-Whitney test or the Wilcoxon test were used to compare intra- and intergroup outcomes andanalysis of variance (ANOVA) for repeated measures, corrected by the Bonferroni post-hoc test,was used to assess postprandial blood glucose.
Results
Only the CCM group showed a reduction in fasting blood glucose. Postprandial blood glucose decreased in the 2nd (p=0.00) and 3rd (p=0.00) gestational trimester in the CCM group, while in the TM group the reduction occurred only in the 2nd trimester (p=0.015). For perinatal outcomes and hypertensive disorders of pregnancy, there were no differences between groups. Cesarean delivery was performed in 82% of the pregnant women and was associated with hypertensive disorders (gestational hypertension or pre-eclampsia; p=0.047).
Conclusion
Both methods of nutritional guidance contributed to the reduction of postprandial glycemia of women and no differences were observed for maternal and perinatal outcomes. However, CCM had a better effect on postprandial glycemia and only this method contributed to reducing fasting blood glucose throughout the intervention. ReBEC Clinical Trials Database The present study was registered in the ReBEC Clinical Trials Database (Registro Brasileiro de Ensaios Clínicos, number RBR-524z9n).
-
Review Article
Oral Iron Supplementation in Pregnancy: Current Recommendations and Evidence-Based Medicine Suplementação oral de ferro na gravidez: recomendações atuais e medicina baseada na evidência
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(10):782-788
12-17-2021
Summary
Review ArticleOral Iron Supplementation in Pregnancy: Current Recommendations and Evidence-Based Medicine Suplementação oral de ferro na gravidez: recomendações atuais e medicina baseada na evidência
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(10):782-788
12-17-2021Views256See moreAbstract
Objective
To review the evidence about universal iron supplementation in pregnancy to prevent maternal anemia.
Methods
Bibliographic research of randomized and controlled clinical trials, meta-analyses, systematic reviews, and clinical guidelines, published between August 2009 and August 2019, using the MeSH terms: iron; therapeutic use; pregnancy; anemia, prevention and control.
Results
We included six clinical guidelines, three meta-analyses and one randomized controlled clinical trial.
Discussion
Most articles point to the improvement of hematological parameters and reduction of maternal anemia risk, with supplementary iron. However, they do not correlate this improvement in pregnant women without previous anemia with the eventual improvement of clinical parameters.
Conclusion
Universal iron supplementation in pregnancy is controversial, so we attribute a SORT C recommendation strength.


