You searched for:"Silvana Maria Quintana"
We found (26) results for your search.Summary
Rev Bras Ginecol Obstet. 2016;38(9):471-476
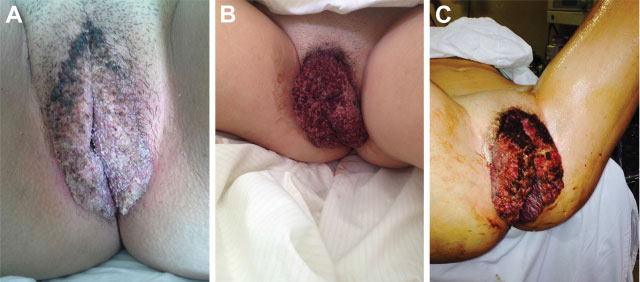
The Buschke-Loewenstein tumor is characterized by excessive growth of verrucous lesions on the genitals and/or perianal region. It is considered benign despite the high rate of recurrence and the possibility of malignant transformation. It is commonly associated with subtypes 6 and 11 of the human papillomavirus (HPV), and host 's immunity plays an important role in the development of the disease. Surgical excision is the recommended treatment in most cases. We present the case of a 16 years old female patient with extensive vulvar lesions successfully treated surgically.
Summary
Rev Bras Ginecol Obstet. 2021;43(8):585-587
Summary
Rev Bras Ginecol Obstet. 2022;44(7):692-700
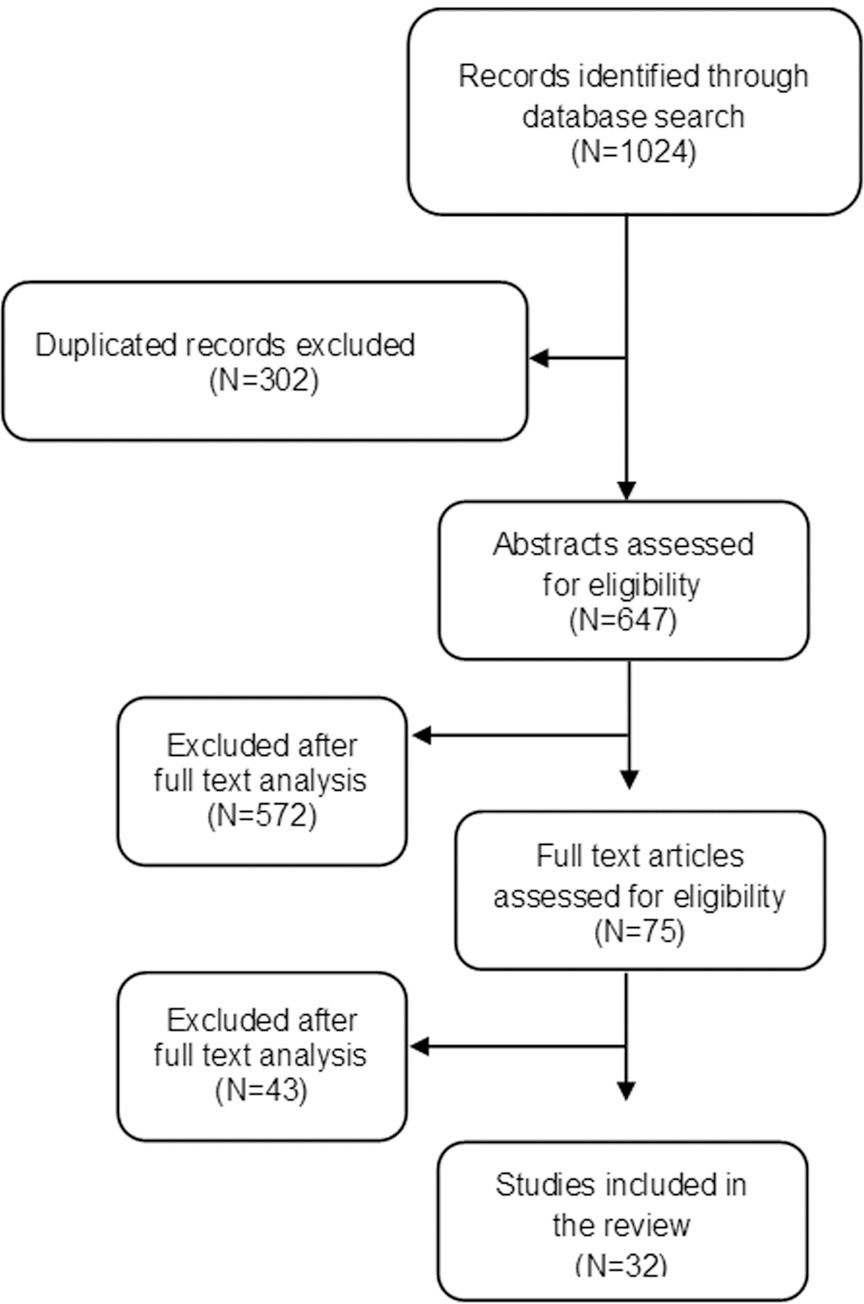
To review concepts, definitions, and findings about fear of childbirth (FOC).
A bibliographic review was carried out through the main scientific databases in 2020.
All 32 articles considered potentially relevant were analyzed. A recent study suggests that the global prevalence of FOC can reach up to 14%. Factors such as parity, gestational age, previous birth experience, age and nationality of the woman seem to influence FOC.
Fear of childbirth could be related to an increased risk of adverse obstetric outcomes such as maternal request for cesarean delivery, preterm birth, prolonged labor, postpartum depression, and post-traumatic stress. These evidence highlight the importance of the discussion regarding this topic.
Summary
Rev Bras Ginecol Obstet. 2005;27(11):698-705
DOI 10.1590/S0100-72032005001100011
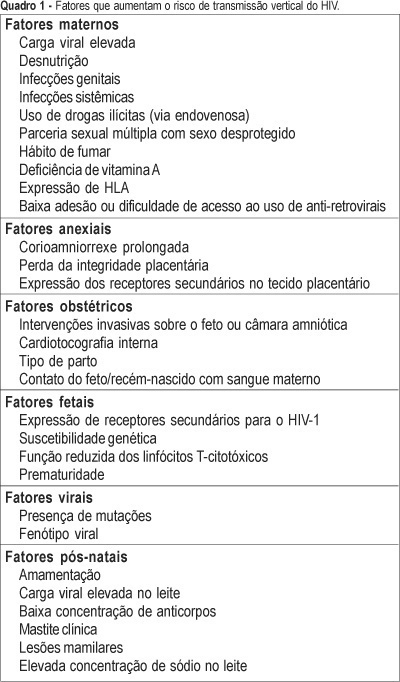
One of the most important advances in the control of the spread of infection with type 1 human immunodeficiency virus (HIV-1) occurred within the context of vertical transmission (VT), with a reduction from levels of more than 40% to levels of less than 3%. Technological progress together with a better physiopathological understanding of this infection has permitted the determination of the situations and factors that increase the rates of perinatal transmission of the virus, indicating which interventions are most adequate for its control. The situations of higher risk for VT of HIV involve maternal, adnexal, obstetrical, fetal, viral, and postnatal factors. Among maternal factors, particularly important is viral load, the major indicator of the risk of this form of transmission. However, despite its relevance, viral load is not the only variable in this equation, with the following factors also playing important roles: use of illicit drugs, multiple sex partners and unprotected sex, malnutrition, smoking habit, advanced maternal disease, and lack af access or compliance with antiretroviral drugs. Among the adnexal factors are prolonged chorion-amniorrhexis, loss of placental integrity, and the expression of secondary receptors in placental tissue. Among the obstetrical factors, it should be remembered that invasive interventions in the fetus or amniotic chamber, internal cardiotocography, type of delivery, and contact of the fetus/newborn infant with maternal blood are also important elements to be controlled. Among the fetal factors are the expression of secondary HIV-1 receptors, genetic susceptibility, reduced cytotoxic T-lymphocyte function, and prematurity. Among the viral factors, mutations and syncytium-inducing strains are believed to be risk factors for VT. Finally, there are postnatal factors represented by an elevated viral load in maternal milk, a low antibody concentration in this fluid, clinical mastitis and nipple lesions, which can be grouped within the context of breast-feeding.
Summary
Rev Bras Ginecol Obstet. 2005;27(12):768-778
DOI 10.1590/S0100-72032005001200010
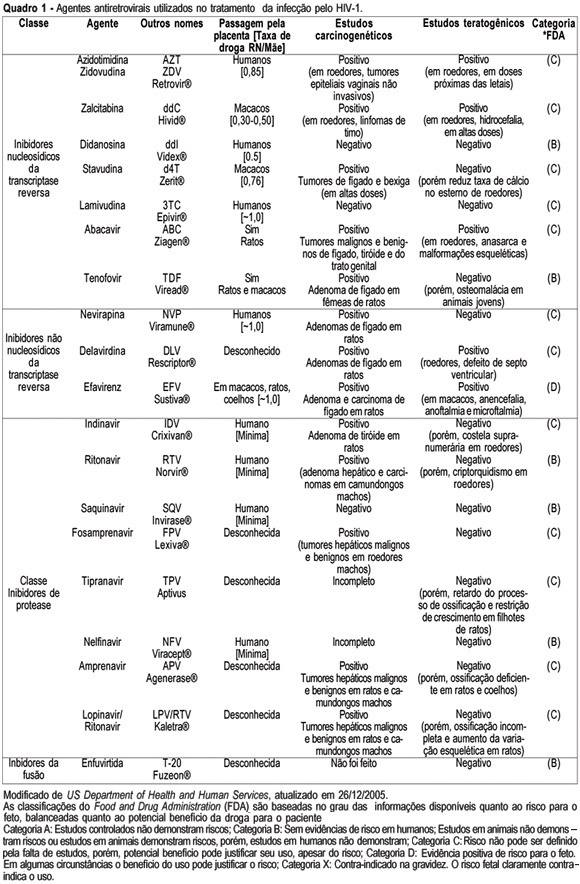
Knowledge about the factors or situations that influence the vertical transmission (VT) of human immunodeficiency type 1 (HIV-1) has led to the implementation of strategies which have promoted a rate decline along the years, from 40% to less than 3% nowadays. One of the major advances in the area has been the prophylactic administration of zidovudine (AZT), in the prenatal phase (oral route), in the predelivery phase (intravenous route) and to the newborn (oral route). This intervention may reduce HIV-1 VT 68%, thus being the most effective isolated strategy used so far. In the chronological sequence of advances, it has been observed that a high viral load is the main risk indicator for this type of transmission. As AZT does not reduce the viral load and does not control the residual rate observed in HIV-1 VT, the use of prophylactic schemes using three antiretroviral drugs has been encouraged. Elective caesarean section completes the range of obstetric strategies with major impact on the reduction of HIV-1 VT. Its effectiveness is linked to the observation of the criteria for its indication: viral load assessed after the 34th week of pregnancy with levels over 1000 copies/mL, gestation over 38 weeks confirmed by ultrasonography, intact chorioamniotic membranes, and performed before labor has started. In cases where normal delivery is indicated, it should be remembered that prolonged chorioamniorrhexis, invasive manipulation of the fetus, delivery with instruments and episiotomy are situations to be avoided. Among the postnatal interventions considered important for the reduction of HIV-1 VT are: pediatric reception (this should be done by trained professionals, avoiding microtraumas in the mucosa during the sucking maneuvers, use of neonatal AZT (for a period of six weeks) and bottle feeding. Special attention should be given to the orientation for the mother, in order to prevent acute infection by HIV-1 in this period, what would markedly increase virus VT rate.
Summary
Rev Bras Ginecol Obstet. 2021;43(2):81-83