Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo21
We conducted a meta-analysis of randomized clinical trials evaluating the clinical effects of ferric carboxymaltose therapy compared to other intravenous iron in improving hemoglobin and serum ferritin in pregnant women. We also assessed the safety of ferric carboxymaltose vs. other intravenous iron.
EMBASE, PubMed, and Web of Science were searched for trials related to ferric carboxymaltose in pregnant women, published between 2005 and 2021. We also reviewed articles from google scholar. The keywords "ferric carboxymaltose," "FCM," "intravenous," "randomized," "pregnancy," "quality of life," and "neonatal outcomes" were used to search the literature. The search was limited to pregnant women.
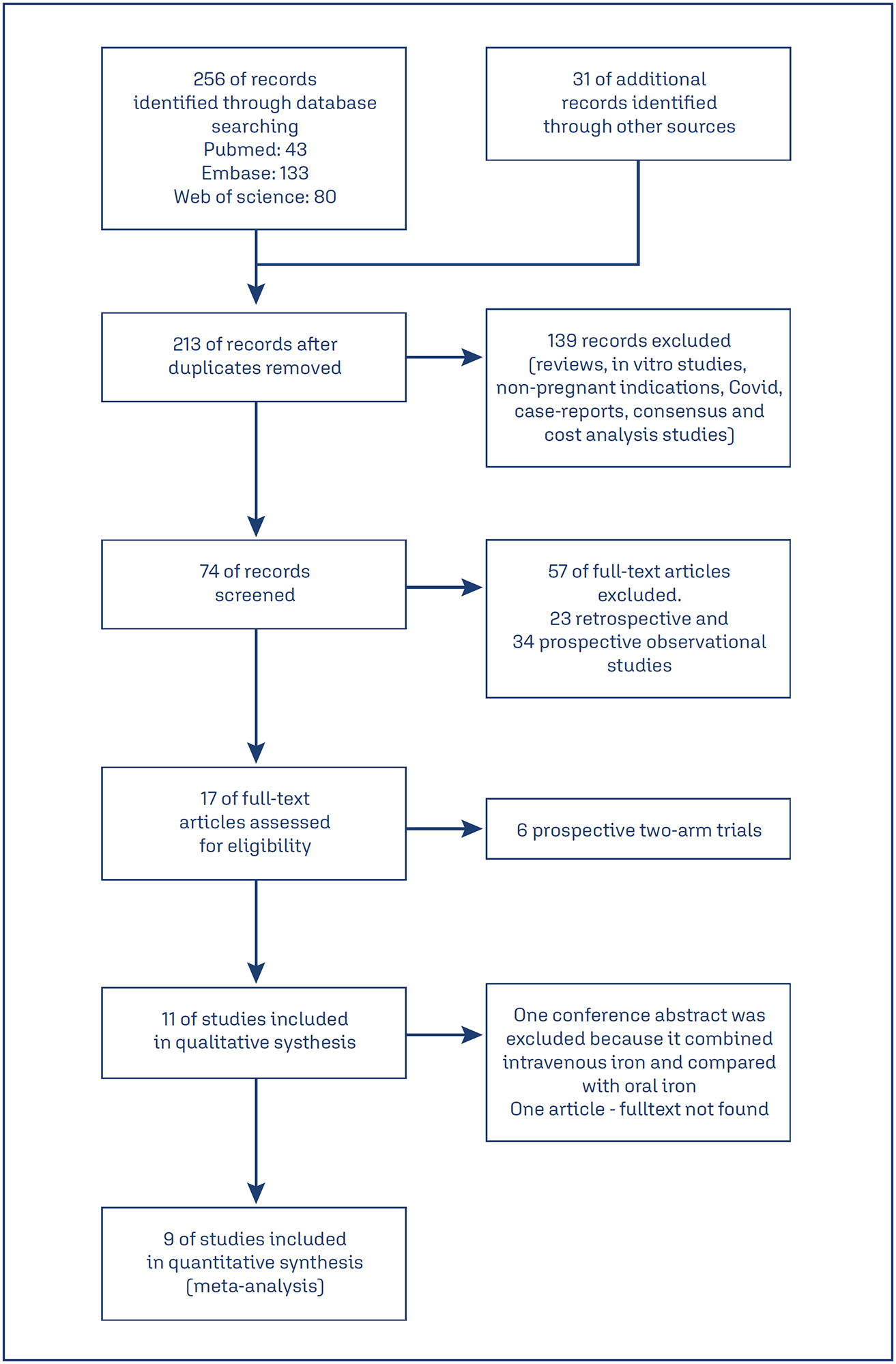
Studies related to ferric carboxymaltose in pregnancy were scanned. Observational studies, review articles, and case reports were excluded. Randomized studies in pregnant women involving ferric carboxymaltose and other intravenous iron formulations were shortlisted. Of 256 studies, nine randomized control trials were selected.
Two reviewers independently extracted data from nine selected trials
The final effect size for increase in hemoglobin after treatment was significant for ferric carboxymaltose vs. iron sucrose/iron polymaltose (standard mean difference 0.89g/dl [95% confidence interval 0.27,1.51]). The final effect size for the increase in ferritin after treatment was more for ferric carboxymaltose vs. iron sucrose/iron polymaltose (standard mean difference 22.53µg/L [-7.26, 52.33]). No serious adverse events were reported with ferric carboxymaltose or other intravenous iron.
Ferric carboxymaltose demonstrated better efficacy than other intravenous iron in increasing hemoglobin and ferritin levels in treating iron deficiency anemia in pregnant women.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo1
To evaluate the association between clinical and imaging with surgical and pathological findings in patients with suspected neuroendocrine tumor of appendix and/or appendix endometriosis.
Retrospective descriptive study conducted at the Teaching and Research Institute of Hospital Israelita Albert Einstein, in which medical records and databases of patients with suspected neuroendocrine tumor of appendix and/or endometriosis of appendix were analyzed by imaging.
Twenty-eight patients were included, all of which had some type of appendix alteration on the ultrasound examination. The pathological outcome of the appendix found 25 (89.3%) lesions compatible with endometriosis and three (10.7%) neuroendocrine tumors. The clinical findings of imaging and surgery were compared with the result of pathological anatomy by means of relative frequency.
It was possible to observe a higher prevalence of appendix endometriosis when the patient presented more intense pain symptoms. The image observed on ultrasound obtained a high positive predictive value for appendicular endometriosis.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo4
To classify the bibliometric indicators of online scientific research on placentophagy.
A bibliometric study was conducted to quantify the scientific production of authors and institutions with the aim of highlighting the growth and impact of these publications nationally and internationally. The Bradford Law, network maps, and textual statistics were used, with searches conducted in libraries and databases in October 2021.
The sample consisted of 64 articles, whose primary authors were associated with 49 institutions, and mostly with degrees in anthropology. The United States of America was the country that published the most papers on the theme, and most studies were reviews with individual production. Through the term analysis, it was found that the predominant themes regarding placentophagy were the following: Alternative therapy for women's health, methodologies used for research in this area, period of placenta ingestion (postpartum period), and its benefits.
The bibliometric indicators found are essential for the development of future research.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo6
BI-RADS® is a standardization system for breast imaging reports and results created by the American College of Radiology to initially address the lack of uniformity in mammography reporting. The system consists of a lexicon of descriptors, a reporting structure with final categories and recommended management, and a structure for data collection and auditing. It is accepted worldwide by all specialties involved in the care of breast diseases. Its implementation is related to the Mammography Quality Standards Act initiative in the United States (1992) and breast cancer screening. After its initial creation in 1993, four additional editions were published in 1995, 1998, 2003 and 2013. It is adopted in several countries around the world and has been translated into 6 languages. Successful breast cancer screening programs in high-income countries can be attributed in part to the widespread use of BI-RADS®. This success led to the development of similar classification systems for other organs (e.g., lung, liver, thyroid, ovaries, colon). In 1998, the structured report model was adopted in Brazil. This article highlights the pioneering and successful role of BI-RADS®, created by ACR 30 years ago, on the eve of publishing its sixth edition, which has evolved into a comprehensive quality assurance tool for multiple imaging modalities. And, especially, it contextualizes the importance of recognizing how we are using BI-RADS® in Brazil, from its implementation to the present day, with a focus on breast cancer screening.