Summary
Rev Bras Ginecol Obstet. 2008;30(2):87-92
DOI 10.1590/S0100-72032008000200007
PURPOSE: to test a therapeutic approach using atosiban for tocolysis, evaluating its safety and maternal and fetal side effects. METHODS: prospective study with 80 pregnant women with preterm labor admitted for tocolysis. Inclusion criteria: singleton pregnancy, regular uterine activity, cervical dilatation between 1 to 3 cm, cervical enfacement greater than 50%, 23 to 33 weeks and six days of gestational age, intact membranes, amniotic fluid index between 5 and 25, no maternal, fetal or placental diseases, no fetal growth restriction, no cervical incompetence, no fever. Exclusion criteria: chorioamnionitis or fever during tocolysis. Atosiban group: women received 6.75 mg atosiban iv in bolus, 300 mcg/min for three hours, then 100 mcg/min for three hours and thirty minutes. If uterine activity persisted, it was maintained iv infusion of 100 mcg/min for 12.5 hand that so for as long as 45 hours. Control group: women received terbutaline (five ampoules, 500 mL crystalloid solution) iv infusion, 20 mL/h. If uterine activity persisted, infusion velocity was raised (20 mL/h) until uterine contractions were absent. The dose was maintained for 24 hours. RESULTS: gestational age at birth was 29 weeks and five days to 40 weeks and six days. In atosiban group, the proportion of women who had not delivered at 48 hours was 97.5%, mean interval between tocolysis and birth of 28.2 days. In control group, birth occurred before 48 hours in 22.5% of the cases; mean interval between tocolysis and birth of 5.3 days. Maternal side effects were observed in 27.5% of cases of the atosiban group, none with tachycardia, dyspnea or tachypnea. In the control group, 75% of the cases referred palpitations, tachycardia, tachypnea or headache (drug infusion was interrupted in four cases). Fetal tachycardia was observed in 22.5% of the cases (n=9). No early neonatal death was observed. CONCLUSIONS: the therapeutic approach used showed to be effective for tocolysis, with low incidence of maternal, fetal and neonatal side effects.
Summary
Rev Bras Ginecol Obstet. 2008;30(2):93-100
DOI 10.1590/S0100-72032008000200008
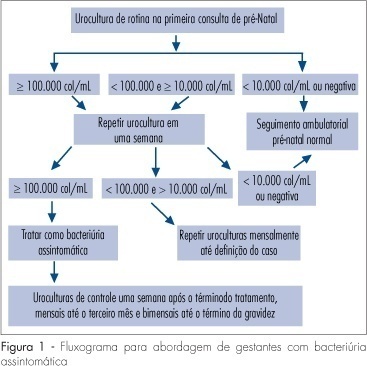
Several factors cause urinary tract infection (UTI) to be a relevant complication of the gestational period, aggravating both the maternal and perinatal prognosis. For many years, pregnancy has been considered to be a factor predisposing to all forms of UTI. Today, it is known that pregnancy, as an isolated event, is not responsible for a higher incidence of UTI, but that the anatomical and physiological changes imposed on the urinary tract by pregnancy predispose women with asymptomatic bacteriuria (AB) to become pregnant women with symptomatic UTI. AB affects 2 to 10% of all pregnant women and approximately 30% of these will develop pyelonephritis if not properly treated. However, a difficult to understand resistance against the identification of AB during this period is observed among prenatalists. The diagnosis of UTI is microbiological and it is based on two urine cultures presenting more than 10(5) colonies/mL urine of the same germ. Treatment is facilitated by the fact that it is based on an antibiogram, with no scientific foundation for the notion that a pre-established therapeutic scheme is an adequate measure. For the treatment of pyelonephritis, it is not possible to wait for the result of culture and previous knowledge of the resistance profile of the antibacterial agents available for the treatment of pregnant women would be the best measure. Another important variable is the use of an intravenous bactericidal antibiotic during the acute phase, with the possibility of oral administration at home after clinical improvement of the patient. At our hospital, the drug that best satisfies all of these requirements is cefuroxime, administered for 10-14 days. Third-generation cephalosporins do not exist in the oral form, all of them involving the inconvenience of parenteral administration. In view of their side effects, aminoglycosides are considered to be inadequate for administration to pregnant women. The inconsistent insinuation of contraindication of monofluorinated quinolones, if there is an indication, norfloxacin is believed to be a good alternative to cefuroxime. In cases in which UTI prophylaxis is indicated, chemotherapeutic agents are preferred, among them nitrofurantoin, with care taken to avoid its use at the end of pregnancy due to the risk of kernicterus for the neonate.