Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo79
To evaluate the maternal outcomes in women with postpartum depression using zuranolone, the first oral medication indicated to treat postpartum depression.
We conducted a systematic search in September 2023, on Pubmed, Embase and Cochrane Trials. We included randomized controlled trials comparing the effectiveness and safety of zuranolone versus placebo in women with postpartum depression. No time or language restrictions were applied. 297 results were retrieved, of which 11 papers were selected and fully reviewed by two authors. Review Manager 5 was used for statistical analysis and Cochrane Risk-of-bias tool for randomized trials was applied for quality assessment.
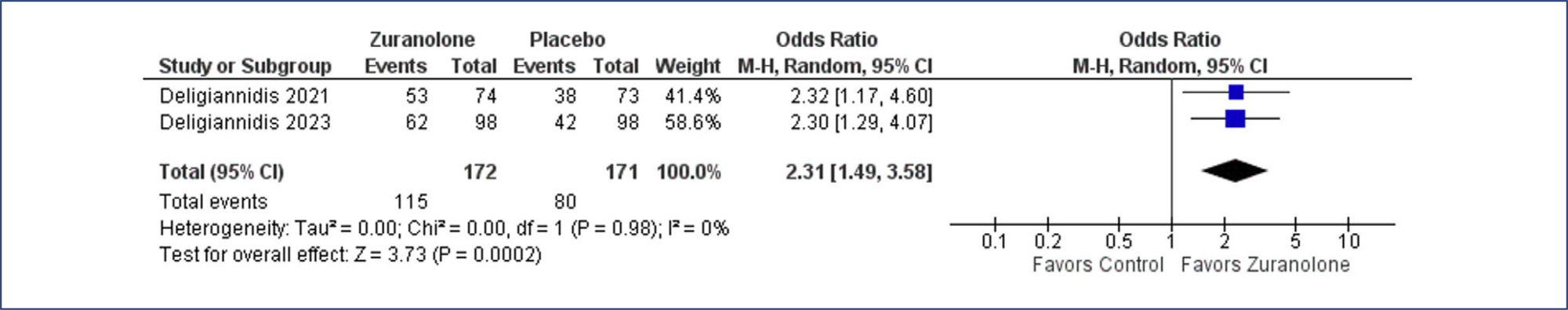
We included 2 studies, with 346 women, of whom 174 (50.2%) were treated with zuranolone. Zuranolone was significantly associated to an improvement of Clinical Global Impression response rate; Hamilton Depression Rating Scale 15 days and 45-day remission, 3-day, 15-day, and 45-day symptom remission, and reduction in the dose of antidepressants. As for safety outcomes, it was noticed that zuranolone increases sedation risk, which can be dose related. No significant differences were found for other adverse events.
These findings suggest that zuranolone might present a safe and effective medication for out-of-hospital treatment of PPD. Sedation effects need to be further assessed.