-
Original Article
Sexuality of Female Spina Bifida Patients: Predictors of a Satisfactory Sexual Function
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(6):467-473
07-27-2021
Summary
Original ArticleSexuality of Female Spina Bifida Patients: Predictors of a Satisfactory Sexual Function
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(6):467-473
07-27-2021Views123See moreAbstract
Objective
To assess the sexual function of women with spina bifida (SB), and to verify the factors that influence their sexual function.
Methods
A cross-sectional study in which a validated female-specific questionnaire was applied to 140 SB female patients from four different cities (Porto Alegre, Brazil; and Barcelona, Madrid, and Málaga, Spain) between 2019 and 2020. The questionnaires collected data on the clinical characteristics of SB, and female sexual function was assessed using the 6-item version of the Female Sexual Function Index (FSFI-6) validated to Portuguese and Spanish.
Results
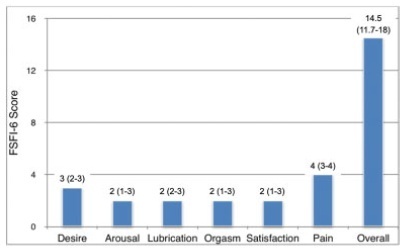
Half of the patients had had sexual activity at least once in the life, but most (57.1%) did not use any contraception method. Sexual dysfunction was present in most (84.3%) patients, and all sexual function domains were impaired compared those of non-neurogenic women. The presence of urinary and fecal incontinence significantly affected the quality of their sexual activity based on the FSFI-6.
Conclusion
The specific clinical aspects of the SB patients, such as urinary and fecal incontinence, should be properly addressed by their doctors, since they are associated with reduced sexual activity and lower FSFI-6 scores in the overall or specific domains. There is also a need to improve gynecological care among sexually-active SB patients, since most do not use any contraceptive methods and are at risk of inadvertent pregnancy.
-
Original Articles
Comparison of Two- and Three-dimensional Ultrasonography in the Evaluation of Lesion Level in Fetuses with Spina Bifida
Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(3):120-126
03-01-2016
Summary
Original ArticlesComparison of Two- and Three-dimensional Ultrasonography in the Evaluation of Lesion Level in Fetuses with Spina Bifida
Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(3):120-126
03-01-2016Views140See morePurpose
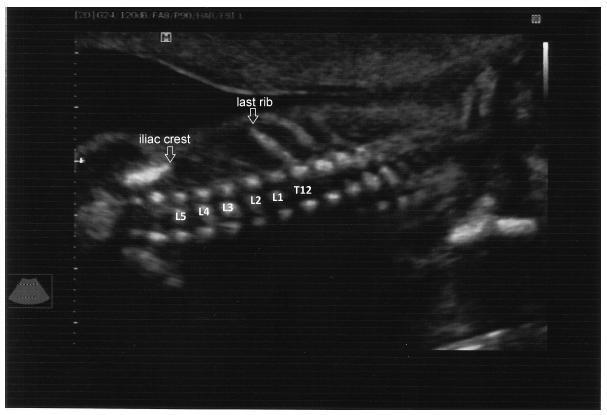
To evaluate the precision of both two- and three-dimensional ultrasonography in determining vertebral lesion level (the first open vertebra) in patients with spina bifida.
Methods
This was a prospective longitudinal study comprising of fetuses with open spina bifida who were treated in the fetal medicine division of the department of obstetrics of Hospital das Clínicas of the Universidade de São Paulo between 2004 and 2013. Vertebral lesion level was established by using both two- and three-dimensional ultrasonography in 50 fetuses (two examiners in each method). The lesion level in the neonatal period was established by radiological assessment of the spine. All pregnancies were followed in our hospital prenatally, and delivery was scheduled to allow immediate postnatal surgical correction.
Results
Two-dimensional sonography precisely estimated the spina bifida level in 53% of the cases. The estimate error was within one vertebra in 80% of the cases, in up to two vertebrae in 89%, and in up to three vertebrae in 100%, showing a good interobserver agreement. Three-dimensional ultrasonography precisely estimated the lesion level in 50% of the cases. The estimate error was within one vertebra in 82% of the cases, in up to two vertebrae in 90%, and in up to three vertebrae in 100%, also showing good interobserver agreement. Whenever an estimate error was observed, both two- and three-dimensional ultrasonography scans tended to underestimate the true lesion level (55.3% and 62% of the cases, respectively).
Conclusions
No relevant difference in diagnostic performance was observed between the two- and three-dimensional ultrasonography. The use of three-dimensional ultrasonography showed no additional benefit in diagnosing the lesion level in the fetuses with spina bifida. Errors in both methods showed a tendency to underestimate lesion level.
-
Artigos Originais
Central nervous system malformations and the presence of the MTHFR-C677T mutation in fetal blood
Revista Brasileira de Ginecologia e Obstetrícia. 2013;35(10):436-441
12-09-2013
Summary
Artigos OriginaisCentral nervous system malformations and the presence of the MTHFR-C677T mutation in fetal blood
Revista Brasileira de Ginecologia e Obstetrícia. 2013;35(10):436-441
12-09-2013DOI 10.1590/S0100-72032013001000002
Views122See morePURPOSE: To evaluate the association between central nervous system (CNS) malformations and the C677T-MTHFR mutation in fetal blood. METHODS: A case-control study was conducted to compare the MTHFR-C677T mutation detected in 78 fetuses with CNS malformations and with 100 morphologically normal fetuses. Genomic DNA was extracted and purified from fetal blood using the Wizard® Genomic DNA Purification Kit (Promega Corp., Madison, WI, USA) according to manufacturer's protocol. The polymerase chain reaction (PCR) was used to assay the thermolabile MTHFR-C677T mutation. The γ² and the Fisher's exact tests were used for descriptive analysis and the Wilcoxon test was used for univariate analysis. Logistic regression analysis was performed to identify which variables were predictors of CNS malformation. RESULTS: Cases and controls were similar regarding maternal characteristics such as age and number of deliveries and abortions. The MTHFR-C677T mutation was detected in 20 cases (25.6%) and in 6 controls in its heterozygous form (OR 10.3; 95%CI 3.3-32.2) and in 6 cases (7.7%) and in 1 control in its homozygous form (OR 12.3; 95%CI 1.3-111.1), and the differences were statistically significant. CONCLUSION: The presence of the MTHFR-C677T mutation in fetal blood was consistent with a higher risk of CNS malformations, both in the heterozygous and homozygous forms.


