Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(2):096-103
07-10-2023
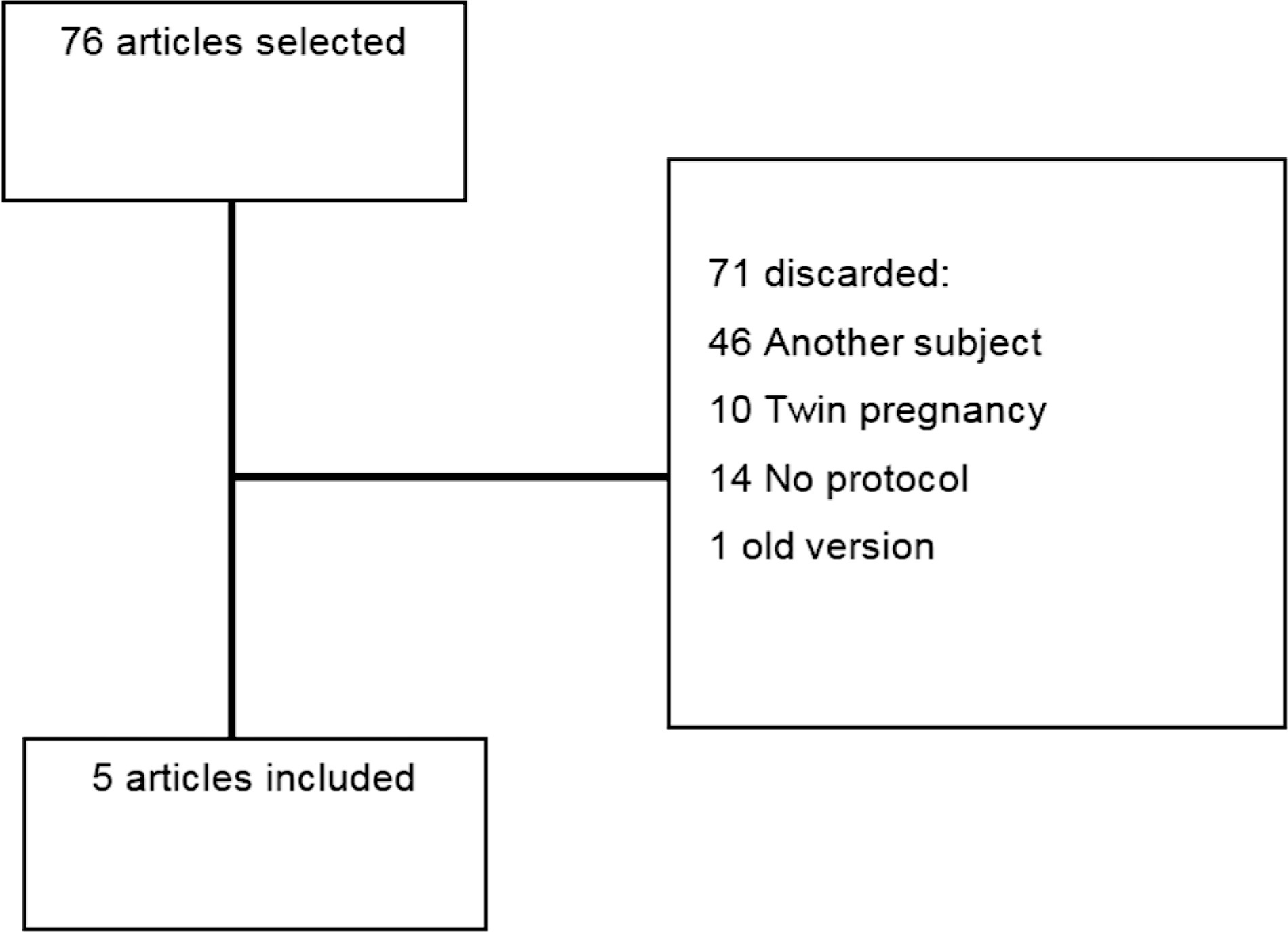
This comprehensive review compares clinical protocols of important entities regarding the management of fetal growth restriction (FGR), published since 2015. Five protocols were chosen for data extraction. There were no relevant differences regarding the diagnosis and classification of FGR between the protocols. In general, all protocols suggest that the assessment of fetal vitality must be performed in a multimodally, associating biophysical parameters (such as cardiotocography and fetal biophysical profile) with the Doppler velocimetry parameters of the umbilical artery, middle cerebral artery, and ductus venosus. All protocols reinforce that the more severe the fetal condition, the more frequent this assessment should be made. The timely gestational age and mode of delivery to terminate the pregnancy in these cases can vary much between the protocols. Therefore, this paper presents, in a didactic way, the particularities of different protocols for monitoring FGR, in order to help obstetricians to better manage the cases.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(6):602-608
08-15-2022
The aim of the present study is to list the published clinical trials on coronavirus disease 2019 (COVID-19) vaccines, to describe the mechanism of action of the identified vaccines, and to identify protocols regarding safety, status, and prioritization of cancer patients for vaccination.
This is a systematic review with a limited literature search conducted by an information specialist; key resources such as PubMed and websites of major cancer organizations were searched. The main search terms were COVID-19, vaccination, cancer, and breast and gynecological cancers.
Cancer patients infected with the new coronavirus are at high risk of complications and death, but we still know little about the risks and benefits of vaccination for COVID-19 in these patients. In an ideal scenario, all cancer patients should have their immunization status updated before beginning treatment, but this is not always possible.
Patients with breast or gynecological cancers who are receiving treatment or are in the 5-year posttreatment period should be included in the priority group for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccination.