Summary
Revista Brasileira de Ginecologia e Obstetrícia. 02-01-2017;39(2):86-89
Case report of a 39-year-old intended mother of a surrogate pregnancy who underwent induction of lactation by sequential exposure to galactagogue drugs (metoclopramide and domperidone), nipple mechanical stimulation with an electric pump, and suction by the newborn. The study aimed to analyze the effect of each step of the protocol on serum prolactin levels, milk secretion and mother satisfaction, in the set of surrogacy. Serum prolactin levels and milk production had no significant changes. Nevertheless, themother was able to breastfeed for four weeks, and expressed great satisfaction with the experience. As a conclusion, within the context of a surrogate pregnancy, breastfeeding seems to bring emotional benefits not necessarily related to an increase in milk production.
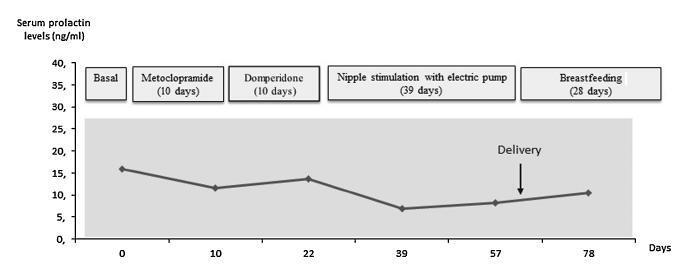
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 03-15-2012;34(2):92-96
DOI 10.1590/S0100-72032012000200009
PURPOSE: To characterize patients with indeterminate values of hyperprolactinemia (PEG test for the identification of macroprolactinemias with recovery between 30 and 65%) (PRLi) or macroprolactinemia (PRLm), in relation to clinical characteristics, such as the presence or absence of symptoms, as well as their intensity and variation, and the presence or absence of central nervous system tumors. METHODS: This is a cross-sectional retrospective survey of records of 24 patients with hyperprolactinemia, in reproductive ages, with prolactin >25 ng/dL. Eleven women with PRLm and 13 with PRLi were included. Records from the two groups were extracted for analysis: age, parity, body mass index, presence of galactorrhea, infertility, and central nervous system tumor. Anthropometrics data were expressed as mean and standard deviation. To compare groups regarding the presence of central nervous system tumor, galactorrhea, as well as infertility we used the Student's t-test. RESULTS: Galactorrhea was more prevalent in patients with PRLi (p=0.01). Seventy percent of women with PRLi presented pituitary tumor (microprolactinoma), whereas this finding was evident in 17% of the PRLm Group (p=0.04). Among the patients with and PRLm PRLi, nine were not investigated with the image of the central nervous system because they have low levels of prolactin (five carriers and four PRLm PRLi). There were no significant differences regarding the occurrence of infertility or irregular menstrual cycles between groups. DISCUSSION: Women with intermediate hyperprolactinemia present more galactorrhea symptoms as well as central nervous system tumors than women with macroprolactinemia.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 04-12-1998;20(9):533-536
DOI 10.1590/S0100-72031998000900007
Purpose: to evaluate the effects of tamoxifen (TAM) on plasma levels of estradiol, progesterone, prolactin, luteinizing hormone (LH), follicle-stimulating hormone (FSH) and steroid hormone-binding globulin (SHBG) when given to premenopausal women in the doses of 10 and 20 mg/day for 22 days. Patients and Methods: a randomized double-blind study was performed with 43 premenopausal eumenorrheic women. The patients were divided into three groups: A (N = 15, placebo); B (N = 15, TAM 10 mg/day) and C (N = 13, 20 mg/day). They started taking an oral dose of TAM or placebo on the very first day of the menstrual cycle. Two hormone determinations were performed, both on the 22nd day of the menstrual cycle: the first in the cycle that preceded the use of the drug and the second, in the following cycle, after 22 days of using the medication. We used the Levine and Student tests in order to evaluate the homogeneity of the sample and the variation of the hormone determinations respectively. Results:serum levels of estradiol, progesterone and SHBG increased significantly in groups B and C. In group C, we also observed increase in serum level of FSH (p < 0.0045) and a fall in prolactin level (p < 0.0055). Conclusions: TAM promoted a significant increase in serum concentrations of estradiol, progesterone and SHBG either in the doses of 10 or 20 mg/day. However, significant increase in FSH and decrease in prolactin were obtained only with the dose of 20 mg/day.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 09-02-2004;26(6):463-469
DOI 10.1590/S0100-72032004000600007
OBJECTIVE: to evaluate serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the pill-free interval of a combined oral contraceptive containing 20 mg of ethynylestradiol and 75 mg of gestodene. METHODS: thirty-one women from 17 to 36 years old, mean age of 24.5 years old, 19% adolescents, were included. FSH, LH, prolactin (PRL) and estradiol (E2) levels were measured by immunochemoluminescence. Both FSH and LH levels were measured within the last four days of pill intake and on the 7th day of the pill-free interval between two cycles. Hormonal levels were compared by the Student t-test. Comparisons between hormonal and anthropometric data were made by linear regression; values of p < 0.05 were taken as significant. RESULTS: seventy-one percent of women were using the pill for the first time. FSH levels increased from 1.3 to 5.7 mIU/ml between the end of the blister pack and the 7th day of the pill-free interval. LH increased from 0.8 to 4.3 mIU/ml. E2 levels changed from 20.2 to 28.0 pg/ml. The levels of PRL decreased from 12.4 to 10.2 ng/ml. There was no correlation between the changes in gonadotrophin levels and most of the anthropometric parameters in these women, with body mass index < 25 kg/m². CONCLUSION: the gonadotrophin levels detected on the last four days of pill intake were greatly suppressed, recovery of three to four times in amount occurring on the 7th day of the pill-free interval.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 08-04-2004;26(5):405-410
DOI 10.1590/S0100-72032004000500010
OBJECTIVE: to assess the ovarian response of poor responsive patients, submitted to the bromocriptine method. PACIENTS AND METHODS: a prospective clinical trial for the in vitro fertilization (IVF) program was performed in 10 poor responsive patients. Endocrinologically normal ovulatory women under 38 years old, who had previously failed in IVF due to poor response to ovarian stimulation with the traditional protocol, were submitted to the bromocriptine method in 12 cycles. They were given bromocriptine, a dopaminergic agonist, in the preceding cycle in order to stop the prolactin production. When the medication was removed at the beginning of the stimulation cycle, an elevation of seric prolactin by a rebound phenomenon was found. This optimized its seric concentration, improving the quality of oocytes and embryos. Serum prolactin and estradiol concentrations, number of follicles, number and quality of oocytes and cleaved embryos, fertilization and pregnancy rates were analyzed. RESULTS: there was a reduction in the dose of gonadotropin administered and in the duration of ovarian stimulation and an improvement in follicular recruitment, oocyte retrieval, embryo morphology, fertilization, and ongoing pregnancy rates. Fertilization rate was 77.7%, pregnancy rate was 44.4% and live baby rate was 25%. CONCLUSION: this study suggests that the bromocriptine method enhanced follicular recruitment and embryonic development, resulting in increased fertilization and pregnancy rates when compared with the traditional protocol for poor responsive patients. Studies with a large number of patients are necessary to confirm these results.