-
Artigos Originais
The effect of soy dietary supplement and low dose of hormone therapy on main cardiovascular health biomarkers: a randomized controlled trial
Revista Brasileira de Ginecologia e Obstetrícia. 2014;36(6):251-258
06-01-2014
Summary
Artigos OriginaisThe effect of soy dietary supplement and low dose of hormone therapy on main cardiovascular health biomarkers: a randomized controlled trial
Revista Brasileira de Ginecologia e Obstetrícia. 2014;36(6):251-258
06-01-2014DOI 10.1590/S0100-720320140004976
Views146PURPOSE:
To assess the effects of a soy dietary supplement on the main biomarkers of cardiovascular health in postmenopausal women compared with the effects of low-dose hormone therapy (HT) and placebo.
METHODS:
Double-blind, randomized and controlled intention-to-treat trial. Sixty healthy postmenopausal women, aged 40-60 years, 4.1 years mean time since menopause were recruited and randomly assigned to 3 groups: a soy dietary supplement group (isoflavone 90mg), a low-dose HT group (estradiol 1 mg plus noretisterone 0.5 mg) and a placebo group. Lipid profile, glucose level, body mass index, blood pressure and abdominal/hip ratio were evaluated in all the participants at baseline and after 16 weeks. Statistical analyses were performed using the χ2 test, Fisher's exact test, Kruskal-Wallis non-parametric test, analysis of variance (ANOVA), paired Student's t-test and Wilcoxon test.
RESULTS:
After a 16-week intervention period, total cholesterol decreased 11.3% and LDL-cholesterol decreased 18.6% in the HT group, but both did not change in the soy dietary supplement and placebo groups. Values for triglycerides, HDL-cholesterol, glucose level, body mass index, blood pressure and abdominal/hip ratio did not change over time in any of the three groups.
CONCLUSION:
The use of dietary soy supplement did not show any significant favorable effect on cardiovascular health biomarkers compared with HT. Clinical Trial Registry: The trial is registered at the Brazilian Clinical Trials Registry (Registro Brasileiro de Ensaios Clínicos - ReBEC), number RBR-76mm75.
Key-words Biological markersEstrogen replacement therapyLipid profileMenopausePhytoestrogensPlacebosRisk factorsSoy foodSee more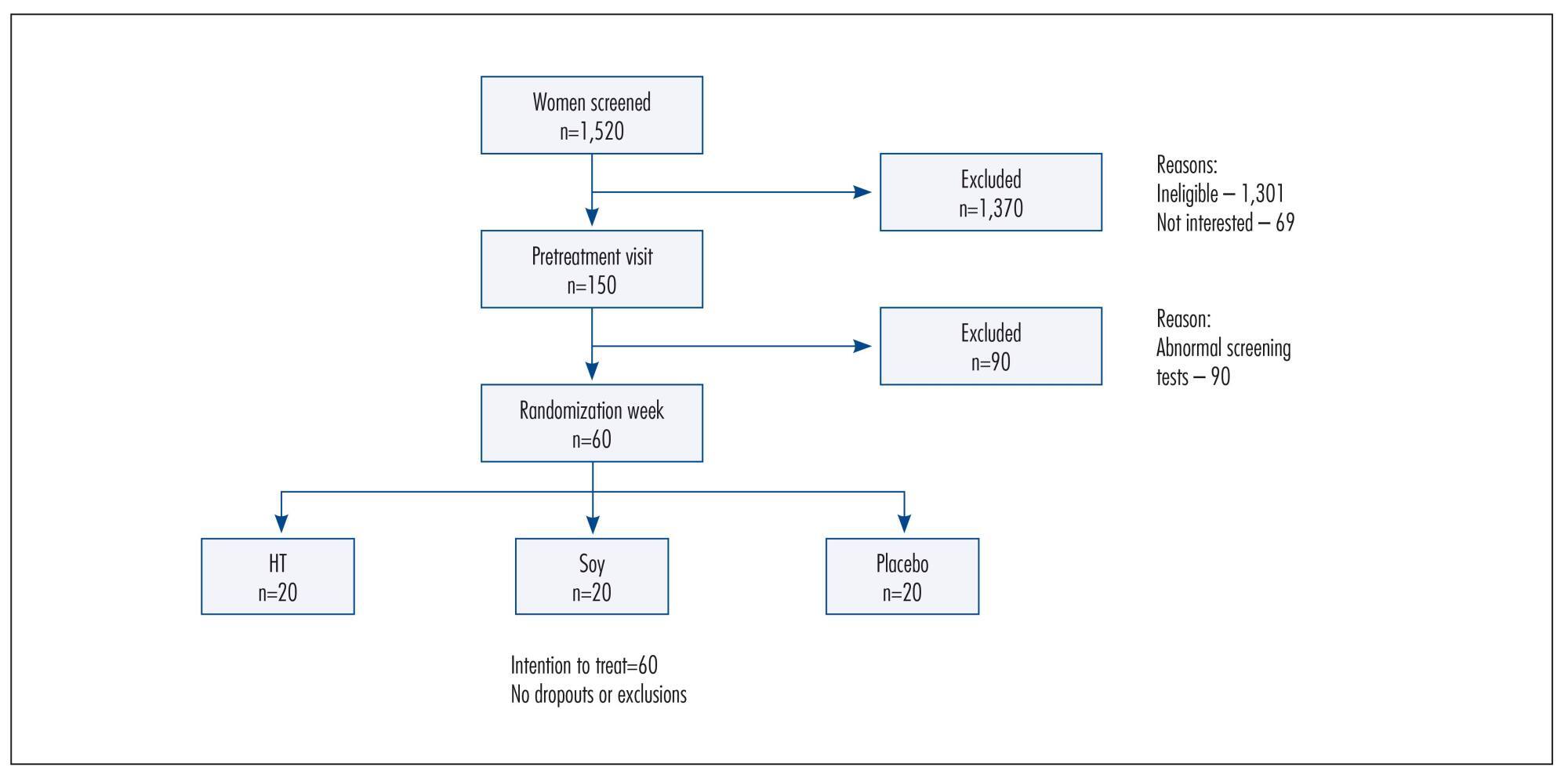
-
Artigos Originais
Tibolone’s effect on retinal and ophthalmic arteries flowmetry
Revista Brasileira de Ginecologia e Obstetrícia. 2008;30(11):537-543
01-12-2008
Summary
Artigos OriginaisTibolone’s effect on retinal and ophthalmic arteries flowmetry
Revista Brasileira de Ginecologia e Obstetrícia. 2008;30(11):537-543
01-12-2008DOI 10.1590/S0100-72032008001100002
Views109PURPOSE: to evaluate the effect of tibolone use on dopplervelocimetric parameters of ophthalmic and retinal arteries. METHODS: clinical, prospective, longitudinal, randomized, placebo-controlled, triple-blind study, in which among 100 menopausal women, 50 have used 2.5 mg of the active principle tibolone (Tib Group) and 50, placebo as a means to form the control-group (Plac Group). In the Tib Group, 44 of the 50 women returned after 84 days to finish the exams, and in the Plac Group, 47. The ophthalmic and retinal arteries were studied to determine the resistance index (RI), the pulsatility index (PI) and the systole/diastole ratio (S/D). Assessments have been done before and 84 days after medication. The t-Student test has been used for the comparison of means between the groups in independent samples, as well as for within-group comparisons in dependent samples. RESULTS: in both groups, the women's characteristics were similar in age, menopause duration, body mass index, arterial blood pressure, deliveries and cardiac rate. The Tib Group presented the following values in the ophthalmic artery: RI(pre)=0.71±0.05, RI(post)0.72±0.08 (p=0.43); PI(pre)=1.29±0.22, PI(post)=1.30±0.25 (p=0.4) and S/D(pre)=3.49±0.77, SD(post)=3.65±0.94 (p=0.32). In the retinal artery, the following values have been found: RI(pre)=0.67±0.09, RI(post)=0.69±0.10 (p=0.7); PI(pre)=1.20±0.29, PI(post)=1.22±0.3 (p=0.2) and SD(pre)=3.29±0.95, SD(post)=3.30±1.07 (p=0.3). Also, the tibolone and control groups did not show any significant difference in regard to the above indexes in the end of the study. CONCLUSIONS: the 2.5 mg dose of tibolone had no effect on the Doppler velocimetry indexes of the ophthalmic and retinal arteries.
Key-words Laser-doppler flowmetryNorpregnanesOphthalmic arteryPlacebosRandomized controlled trialsRetinal arteryUltrasonographySee more


