Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(3):140-146
03-01-2016
The aim of this study was to study the effects of Tribulus terrestris on sexual function in menopausal women.
This was a prospective, randomized, double-blind, placebo-controlled clinical trial that included 60 postmenopausal women with sexual dysfunction. The women were divided into two groups, placebo group and Tribulus group, and evaluated by using the Sexual Quotient-female version (SQ-F) and Female Intervention Efficacy Index (FIEI) questionnaires.
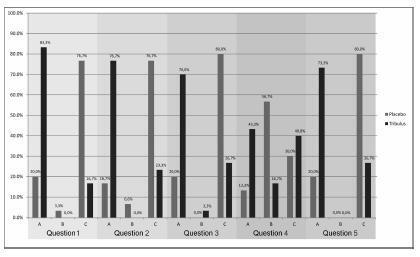
There was no significant difference between the groups in age, age at menopause, civil status, race, and religion. In the evaluation with the SQ-F questionnaire, there were significant differences between the placebo (7.6±3.2) and Tribulus (10.2±3.2) groups in the domains of desire and sexual interest (p d" 0.001), foreplay (3.3±1.5 versus 4.2±1.0) (p d" 0.01), arousal and harmonious interaction with the partner (5.7±2.1 versus 7.2±2.6) (p d" 0.01), and comfort in sexual intercourse (6.5±2.4 versus 8.0±1.9) (p d" 0.01). There was no significant difference between the placebo and Tribulus groups in the domains of orgasm and sexual satisfaction (p = 0.28). In the FIEI questionnaire, there was a significant improvement (p < 0.001) in the domains of vaginal lubrication during coitus and/or foreplay (20 versus 83.3%), sensation in the genitalia during sexual intercourse or other stimuli (16.7 versus 76.7%), sensation in the genital region (20 versus 70%), sexual intercourse and/or other sexual stimulations (13.3 versus 43.3%), and the ability to reach orgasm (20% versus 73.3%). There was no significant difference in adverse effects between the two groups.
After 90 days of treatment, at the doses used, we found Tribulus terrestris to be effective in treating sexual problems among menopausal women.