Summary
Revista Brasileira de Ginecologia e Obstetrícia. 08-29-2022;44(7):654-659
Urodynamic studies (UDSs) are a set of tests that assess the storage and emptying of urine, and they are widely used by gynecologists and urologists in the management of urinary incontinence (UI), despite the discussion about its indications. The objectives of the present study were to verify whether UDSs are routinely used in the conservative and surgical approaches to female UI, their other clinical indications, and to compare the responses of Brazilian gynecologists and urologists.
The present is an opinion survey applied from August 2020 to January 2021 through a semistructured questionnaire about the clinical practice sent by e-mail to all participants. The responses were compared through statistical analyses.
Of the 329 participants, 238 were gynecologists (72.3%) and 91, urologists (27.7%). Most gynecologists (73.5%) and urologists (86.6%) do not request UDSs before the conservative treatment of UI; but UDSs are indicated in the preoperative period of anti-incontinence surgeries. Most participants request UDSs in the initial approach to overactive bladder (gynecologists: 88.2%; urologists: 96.7%), and the urologist has greater chance to request this study (odds ratio [OR] = 3.9). For most participants, it is necessary to request uroculture before the UDSs.
Most Brazilian gynecologists and urologists who participated in the present study do not request UDSs before the conservative treatment of UI, according to national and internacional guidelines, and often request it before the surgical treatment for female UI. The indication of this exam in the initial approach of idiopathic overactive bladder should be reviewed by the participants.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 04-01-2018;40(4):225-231
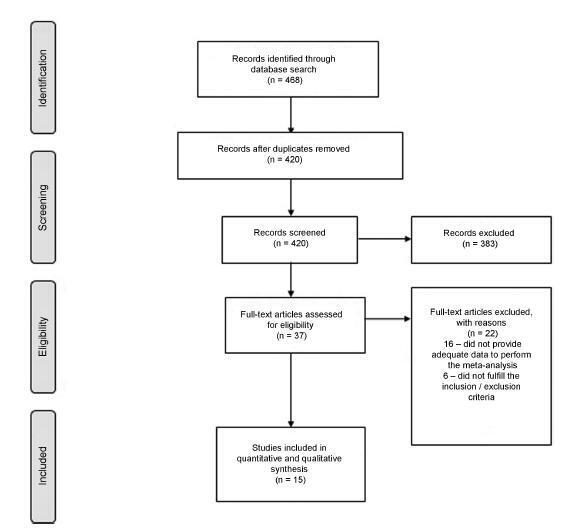
We performed a systematic review and meta-analysis of randomized placebo-controlled trials that studied non-neurogenic overactive bladder patients who were treated with 100 units of onabotulinumtoxinA or placebo. The primary purpose of our study was to evaluate the clinical effectiveness with regard to urinary urgency, urinary frequency, nocturia, and incontinence episodes. Our secondary purpose consisted of evaluating the adverse effects. Our initial search yielded 532 entries. Of these, seven studies met all the inclusion criteria (prospective, randomized, placebo-controlled studies, ≥ 3 points on the Jadad scale) and were selected for analysis. For all primary endpoints, the toxin was more effective than placebo (p < 0.0001; 95% confidence interval [95CI]), namely: urgency (mean difference = -2.07; 95CI = [-2.55-1.58]), voiding frequency (mean difference = - 1.64; 95CI = [-2.10-1.18]), nocturia (mean difference = -0.25; 95CI = [-0.39-0.11]) and incontinence episodes (mean difference = -2.06; 95CI= [-2.60-1.52]). The need for intermittent catheterization and the occurrence of urinary tract infection (UTI) were more frequent in patients treated with onabotulinumtoxinA than in patients treated with placebo (p < 0.0001). Compared with placebo, onabotulinumtoxinA had significantly and clinically relevant reductions in overactive bladder symptoms and is associated with higher incidence of intermittent catheterization and UTI.

Summary
Revista Brasileira de Ginecologia e Obstetrícia. 11-01-2016;38(11):564-575
The overactive bladder (OAB) has a significant negative impact on the quality of life of patients. Antimuscarinics have become the pharmacological treatment of choice for this condition. The objective of this systematic review and meta-analysis is to examine the evidence from randomized clinical trials about the outcomes of the antimuscarinic drugs available in Brazil on OABs. We searched MEDLINE and the Cochrane Central Register of Controlled Trials from the inception of these databases through to September 2015. The primary outcome measures were the mean decrease in urge urinary incontinence episodes and the mean decrease in the frequency of micturition. The results suggest that there is a moderate to high amount of evidence supporting the benefit of using anticholinergic drugs in alleviating OAB symptoms when compared with placebo. It is still not clear whether any of the specific drugs that are available in Brazil offer advantages over the others. These drugs are associated with adverse effects (dry mouth and constipation), although they are not related to an increase in the number of withdrawals.