-
Review Article
Efficacy of Transversus Abdominis Plane Block in the Reduction of Pain and Opioid Requirement in Laparoscopic and Robot-assisted Hysterectomy: A Systematic Review and Meta-analysis
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(1):55-66
02-28-2022
Summary
Review ArticleEfficacy of Transversus Abdominis Plane Block in the Reduction of Pain and Opioid Requirement in Laparoscopic and Robot-assisted Hysterectomy: A Systematic Review and Meta-analysis
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(1):55-66
02-28-2022Views279Abstract
Objective
To summarize the available evidence of TAP Block in efficacy in laparoscopic or robotic hysterectomy.
Data Sources
We searched databases and gray literature for randomized controlled trials in which transversus abdominis plane (TAP) block was compared with placebo or with no treatment in patients who underwent laparoscopic or robot-assisted hysterectomy.
Method of Study
Selection Two researchers independently evaluated the eligibility of the selected articles. Tabulation, Integration, and Results Seven studies were selected, involving 518 patients. Early postoperative pain showed a difference in the mean mean difference (MD): - 1.17 (95% confidence interval [CI]: - 1.87-0.46) in pain scale scores (I2=68%), which was statistically significant in favor of using TAP block, but without clinical relevance; late postoperative pain: DM 0.001 (95%CI: - 0.43-0.44; I2=69%); opioid requirement: DM 0.36 (95%CI: - 0.94-1.68; I2=80%); and incidence of nausea and vomiting with a difference of 95%CI=- 0.11 (- 0.215-0.006) in favor of TAP.
Conclusion
With moderate strength of evidence, due to the high heterogeneity and imbalance in baseline characteristics among studies, the results indicate that TAP block should not be considered as a clinically relevant analgesic technique to improve postoperative pain in laparoscopic or robotic hysterectomy, despite statistical significance in early postoperative pain scale scores.
Key-words laparoscopic hysterectomyOpioidPainrobotic-assisted hysterectomytransversus abdominis plane blockSee more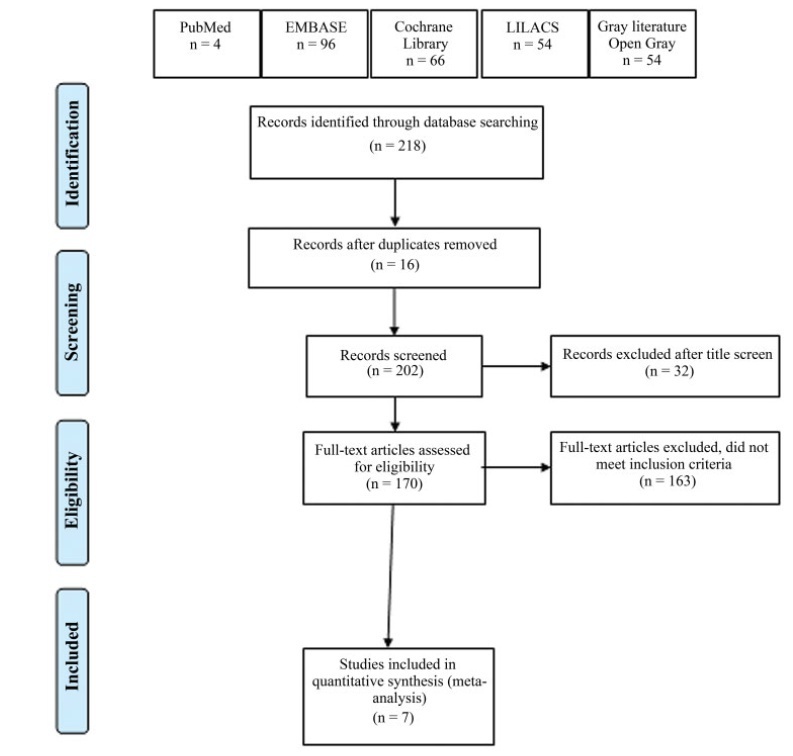
-
Trabalhos Originais
Effects of the association of an opioid with epidural analgesia for labor and delivery
Revista Brasileira de Ginecologia e Obstetrícia. 1998;20(6):325-331
04-11-1998
Summary
Trabalhos OriginaisEffects of the association of an opioid with epidural analgesia for labor and delivery
Revista Brasileira de Ginecologia e Obstetrícia. 1998;20(6):325-331
04-11-1998DOI 10.1590/S0100-72031998000600005
Views59See moreThe purpose of the present study was to evaluate the efficacy and safety of the association bupivacaine with sufentanil for labor and delivery analgesia through a continuous epidural blockade, for both mother and the neonate. A randomized double blind prospective clinical trial was performed including sixty nulliparous women at the Maternity of CAISM/UNICAMP. When requesting analgesia, the women were randomly allocated to two groups: BS, receiving 12.5 mg of bupivacaine with adrenaline plus 30 µg of sufentanil and BP, receiving 12.5 mg of bupivacaine with adrenaline plus placebo. The parameters concerning the quality and duration of analgesia, duration of labor, and also possible effects on the neonate were evaluated. The results showed the superiority of the addition of sufentanil regarding the degree of analgesia during the time of action of the first dose of the local anesthetic. There was no increase in the duration of labor after the onset of analgesia when comparing both groups, nor any difference in the route of delivery. Concerning neonate evaluation, there were no differences between the two groups. It is concluded that the association of 30 µg of sufentanil with the first dose of bupivacaine is safe and efficacious. It improved the quality of analgesia, increased its duration, and did not affect the progress of labor and neonatal outcome.


