Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo29
07-26-2024
Neoadjuvant chemotherapy (NACT) has become the standard of care for patients with triple-negative breast cancer (TNBC) with tumors > 1 cm or positive axillary nodes. Pathologic complete response (pCR) has been used as an endpoint to select patients for treatment scaling. This study aimed to examine the benefit of adding adjuvant capecitabine for TNBC patients who did not achieve pCR after standard NACT in a real-world scenario.
This retrospective cohort study included all patients with TNBC who underwent NACT between 2010 and 2020. Clinicopathological data were obtained from the patient records. Univariate and multivariate analyses were conducted at the 5 years follow-up period.
We included 153 patients, more than half of whom had stage III (58.2%) and high-grade tumors (60.8%). The overall pCR rate was 34.6%, and 41% of the patients with residual disease received adjuvant capecitabine. Disease-specific survival (DSS) among the patients who achieved pCR was significantly higher (p<0.0001). Residual disease after NACT was associated with detrimental effects on DSS. In this cohort, we did not observe any survival benefit of adding adjuvant capecitabine for patients with TNBC subjected to NACT who did not achieve pCR (p=0.52).
Our study failed to demonstrate a survival benefit of extended capecitabine therapy in patients with TNBC with residual disease after NACT. More studies are warranted to better understand the indication of systemic treatment escalation in this scenario.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(12):710-717
02-03-2019
To identify the biomarkers of response to neoadjuvant chemotherapy in early luminal breast cancer.
A cross-sectional study that included all patients with early or locallyadvanced luminal breast cancer submitted to neoadjuvant chemotherapy between 2013 and 2014. Demographic, clinic and pathologic data were retrieved from patient records. The expressions of the estrogen receptor (ER), the progesterone receptor (PR), and Ki67 were analyzed by immunohistochemistry (IHC). The status of the human epidermal growth factor receptor 2 (HER2) was evaluated by IHC and fluorescent in situ hybridization (FISH). Independent predictors of clinic and pathologic response were evaluated by stepwise logistic regression models and receiver operating characteristic (ROC) curve analysis.
Out of 298 patients identified, 115 were included in the analysis. Clinical complete response (cCR) was observed in 43.4% of the patients (49/113), and pathologic complete response (pCR) was observed in 7.1% (8/115) of the patients. The independent predictors of cCR were premenopausal status (p < 0.001), low PR expression (≤ 50% versus > 50%; p = 0.048), and Ki67 expression ≥ 14% (versus < 14%; p = 0.01). Patients with cCR were more commonly submitted to breast conserving surgery (34.7% versus 7.8%; p < 0.001). Increasing cut-off points for Ki67 expression were associated with an increase in specificity and a decrease in sensitivity to identify patients with cCR.
Premenopausal status, lower PR expression and higher Ki67 expression were associated with a higher rate of cCR to neoadjuvant chemotherapy in luminal breast cancer.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(6):280-286
06-01-2016
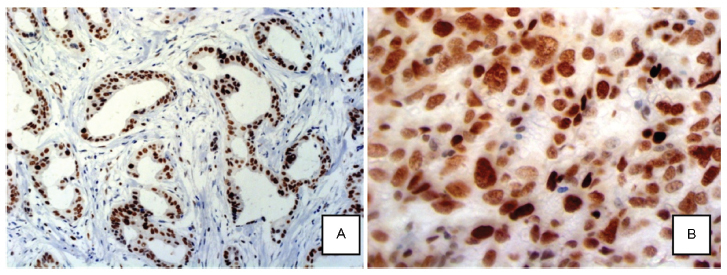
Neoadjuvant chemotherapy (NAC) is the standard treatment for locally advanced breast cancer. However, some tumors will not respond to this treatment due to histological and molecular features. The protein EZH2 (enhancer of zest homolog 2) is a histone methyltransferase that is correlated with poorly differentiated breast carcinomas and aggressive tumor behavior.
The present study evaluated the association between EZH2 expression and response to NAC, and its correlation with HER2 overexpression, estrogen and progesterone receptors (ER, PR) and Ki-67 proliferation index.
A total of 60 patients with locally advanced breast cancer treated with NAC were selected for this study. Twenty-three paraffin blocks had not enough material for tissue resection, and were not evaluated. A tissue microarray based in immunohistochemistry (IHC) analysis of EZH2 was performed for the remaining 37 specimens. Patients were divided into two groups based on response to NAC.
EZH2 expression was significantly associated with markers of poor prognosis such as ER negativity (p = 0.001), PR negativity (p = 0.042) and high Ki-67 proliferation index (p = 0.002). High EZH2 expression was not correlated with the response to NAC.
Our data suggested that EZH2 protein expression may not correlate with the clinical response to NAC. Other studies with more patients are needed to confirm this observation.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2013;35(5):221-225
07-05-2013
DOI 10.1590/S0100-72032013000500006
PURPOSE: We aimed to determine whether clinical examination could adequately ascertain the volume of tissue to be resected during breast-conserving surgery after neoadjuvant therapy. METHODS: We reviewed the clinical reports of 279 patients with histologically diagnosed invasive breast carcinomas treated with neoadjuvant therapy followed by surgery or with primary surgery alone. We estimated volumes of excised tissues, the volume of the tumor mass and the optimal volume required for excision based on 1 cm of clear margins. The actual excess of resected volume was estimated by calculating the resection ratio measured as the volume of the resected specimen divided by the optimal specimen volume. The study endpoints were to analyze the extent of tissue resection and to ascertain the effect of excess resected tissue on surgical margins in both groups of patients. RESULTS: The median tumor diameter was 2.0 and 1.5 cm in the surgery and neoadjuvant therapy groups, respectively. The median volume of resected mammary tissue was 64.3 cm³ in the primary surgery group and 90.7 cm³ in the neoadjuvant therapy group. The median resection ratios in the primary surgery and neoadjuvant therapy groups were 2.0 and 3.3, respectively (p<0.0001). Surgical margin data were similar in both groups. Comparison of the volume of resected mammary tissues with the tumor diameters showed a positive correlation in the primary surgery group and no correlation in the neoadjuvant therapy group. CONCLUSION: Surgeons tend to excise large volumes of tissue during breast-conserving surgery after neoadjuvant therapy, thereby resulting in a loss of the correlation between tumor diameter and volume of the excised specimen.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2011;33(1):43-48
05-17-2011
DOI 10.1590/S0100-72032011000100007
The treatment options for pregnant patients with invasive cervical cancer (ICC) depend on gestational age, clinical stage and the patient's wishes. Some authors have reported cases of neoadjuvant chemotherapy followed by radical surgery in these patients. The aim of this paper was to revisit this subject and to add a new case and review the literature. We report the case of a 30 year-old woman in the 24th week of gestation. She was diagnosed with ICC (squamous cell carcinoma grade 2), stage IIB (International Federation of Gynecology and Obstetrics - FIGO). Nulliparous, the patient refused to interrupt the pregnancy. After meticulous counseling, the patient accepted treatment with neoadjuvant chemotherapy (cisplatin 75 mg/m² and vincristine 1 mg/m²) and subsequent evaluation of radical surgery concomitant to a cesarean section. Four complete cycles of chemotherapy were administered without delays or significant adverse effects. A few days before the date scheduled for surgery, the patient was admitted in advanced labor (37th week of gestation). Due to tumor clinical response, the obstetric team decided to monitor the labor, and the patient gave birth to a male newborn (2,450 g) uneventfully. Radical surgery was performed three days after birth, and histopathology analysis revealed carcinoma confined to the cervix without lymphatic involvement. Mother and son are in good general condition 12 months after delivery. Cisplatin-based chemotherapy during the second or third trimester of pregnancy appears to be a safe option for patients who do not wish to interrupt a pregnancy while awaiting fetal maturity. However, additional studies are needed to confirm the prognosis and assure the safety of newborns and patients.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2007;29(1):18-26
04-04-2007
DOI 10.1590/S0100-72032007000100004
PURPOSE: to evaluate the loco-regional response to primary chemotherapy in patients with breast cancer at stages II and III. METHODS: a retrospective and analytical clinical study carried out in 97 patients with an average age of 52.2 years old, with breast cancer at stages II and III, attended from January 1993 to December 2004, and submitted to 3 to 4 cycles of primary chemotherapy with 5-fluorouracil - 500 mg/m2, epirubicin - 50 mg/m2 and cyclophosphamide - 500 mg/m2 or doxorubicin - 50 mg/m2 e cyclophosphamide - 500 mg/m2, and then to loco-regional surgical conservative or radical surgical treatment. Chi-square and Fisher’s exact tests were used to study the association among the variables (age, menopausal state, pre-chemotherapy tumoral volume, axillary condition, stage, therapeutic scheme and number of cycles), while Pearson’s correlation coefficient was used for the quantitative variables (tumoral volume according to the anatomo-pathological study and the post-chemotherapy clinical tumoral volume. The significance level was 5%. RESULTS: there were 56.8% of cases at stage II and 43.2% at stage III. Approximately 50% of the patients received FEC50 and 50% AC. Objective clinical response with primary chemotherapy was obtained in 64.9% of the cases. Full clinical response occurred in 12.3% of patients, while full pathological response occurred in 10.3% of the cases. CONCLUSIONS: there was a statistically significant correlation between the number of cycles and the response to primary chemotherapy. Patients who received 4 cycles had better response than those who received 3 cycles. There was also a statistically significant concordance between the evaluation through clinical examination of the response to primary chemotherapy and the pathological findings. No statistically significant correlation was observed concerning age, menopausal status, tumoral volume, and pretreatment of axillary damage.