-
Review Article12-04-2024
Metformin versus insulin in gestational diabetes mellitus: a systematic review
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo89
Abstract
Review ArticleMetformin versus insulin in gestational diabetes mellitus: a systematic review
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo89
Views340See moreAbstract
Objective:
The aim of this study is to assess the use of metformin with or without insulin for the treatment of Gestational Diabetes Mellitus compared to insulin alone.
Data sources:
This article consists of a systematic review of randomized clinical trials. The searches were carried out on MEDLINE including 7 studies, between 2010 to 2021.
Study selection:
Randomized clinical trials comparing metformin and insulin written in English, Spanish or Portuguese, with no time limit, were included.
Data collection:
Data was extracted from all the 7 articles and compared statistically when possible. Whenever data was not available or couldn't be statistically compared, the main results were described in detail.
Data synthesis:
Insulin alone is not superior than metformin with or without insulin on gestational diabetes mellitus.
Conclusion:
There is a potential viability of using metformin as an alternative compared to insulin alone in the treatment of Gestational Diabetes Mellitus. However, all assessed outcomes have a very low level of certainty of evidence and more studies are necessary to support these findings.
-
Original Article02-03-2019
Factors Associated with the Need for Insulin as a Complementary Treatment to Metformin in Gestational Diabetes Mellitus
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(12):697-702
Abstract
Original ArticleFactors Associated with the Need for Insulin as a Complementary Treatment to Metformin in Gestational Diabetes Mellitus
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(12):697-702
Views229See moreAbstract
Objective
To evaluate the factors associated with the need for insulin as a complementary treatment to metformin in pregnant women with gestational diabetes mellitus (GDM).
Methods
A case-control study was performed from April 2011 to February 2016 with pregnant women with GDM who needed complementary treatments besides diet and physical exercise. Those treated with metformin were compared with those who, in addition to metformin, also needed the combination with insulin. Maternal characteristics and glycemic control were evaluated. Multinomial logistic regression models were developed to evaluate the influence of different therapies on neonatal outcomes.
Results
A total of 475 pregnant women who needed pharmacological therapy were evaluated. Of these, 366 (77.05%) were submitted to single therapy with metformin, and 109 (22.94%) needed insulin as a complementary treatment. In the analysis of the odds ratio (OR), fasting glucose (FG)<90 mg/dL reduced the odds of needing the combination (OR: 0.438 [0.235-0.815]; p=0.009], as well as primiparity (OR: 0.280 [0.111-0.704]; p=0.007]. In obese pregnant women, an increased chance of needing the combination was observed (OR: 2,072 [1,063-4,039]; p=0,032).
Conclusion
Obesity resulted in an increased chance of the mother needing insulin as a complementary treatment to metformin, while FG<90 mg/dL and primiparity were protective factors.
-
Review Article11-01-2018
Evaluation of Preeclampsia Results after Use of Metformin in Gestation: Systematic Review and Meta-analysis
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(11):713-721
Abstract
Review ArticleEvaluation of Preeclampsia Results after Use of Metformin in Gestation: Systematic Review and Meta-analysis
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(11):713-721
Views201See moreAbstract
Objective
Does the use of metformin have an influence on the outcomes of preeclampsia (PE)?
Sources of Data
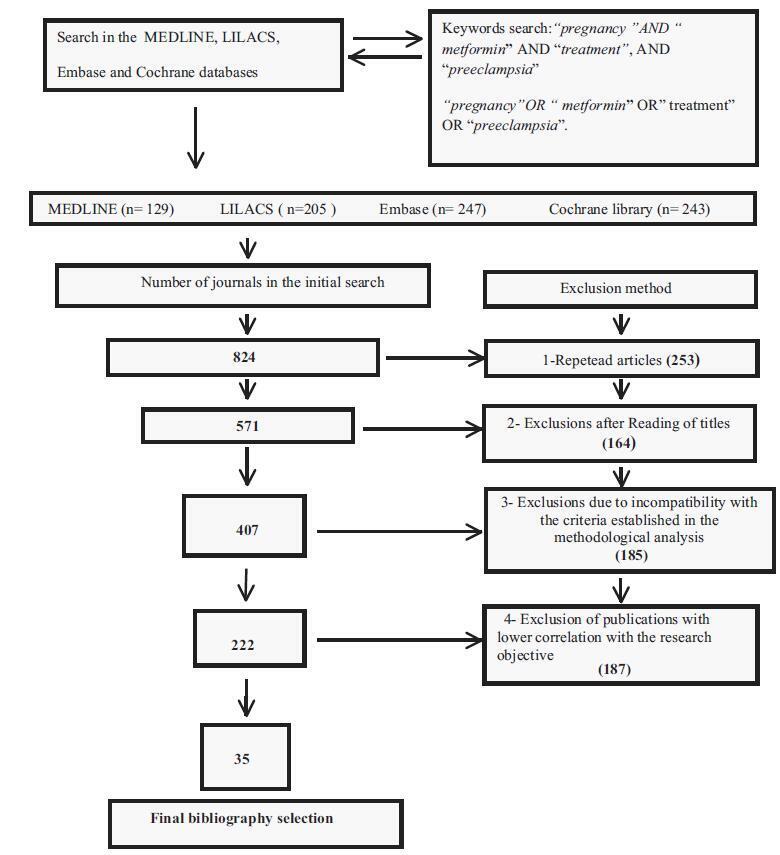
The descriptors pregnancy, metformin, treatment, and preeclampsia associated with the Boolean operators AND and OR were found in the MEDLINE, LILACS, Embase and Cochrane databases. A flowchart with exclusion criteria and inclusion strategy using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol, and eligibility criteria was used. Data were extracted regarding the type of study, the applied dosage, treatment time, segment, bias risks, and the Patient, Intervention, Comparison and Outcome (PICO) strategy to identify the quality of the study.
Selection of Studies
Total number of journals in the initial search (n= 824); exclusions from repeated articles on different search engines (n= 253); exclusions after reading the titles, when the title had no correlations with the proposed theme (n= 164); exclusions due to incompatibility with the criteria established in the methodological analysis (n= 185), exclusion of articles with lower correlation with the objective of the present study (n= 187); and final bibliographic selection (n= 35).
Data Collection
At first, a systematic review of the literature was performed. Subsequently, from the main selection, randomized and non-randomized trials with metformin that presented their results in absolute and relative numbers of PE outcomes were selected. The variables were treated statistically in the meta-analysis with the Review Manager software (RevMan), version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration. Denmark in the Hovedistaden region.
Synthesis of Data
The study showed that metmorfin presented greater preventive effects for pregnancy-induced hypertension and was less effective for PE.
Conclusion
Metformin may gain place in preventive treatments for PE, once the dosages, the gestational age, and treatment time are particularly evaluated. A methodological strategy with an improved perspective of innovative and/or carefully progressive dosages during pregnancy to avoid side effects and the possibility of maternal-fetal risks is suggested.
-
Original Article04-01-2018
Effectiveness of Metformin in the Prevention of Gestational Diabetes Mellitus in Obese Pregnant Women
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(4):180-187
Abstract
Original ArticleEffectiveness of Metformin in the Prevention of Gestational Diabetes Mellitus in Obese Pregnant Women
Revista Brasileira de Ginecologia e Obstetrícia. 2018;40(4):180-187
Views225See moreAbstract
Objective
To assess the effectiveness of metformin in the incidence of gestational diabetes mellitus (GDM) in obese pregnant women attending a public maternity hospital in Joinville, Santa Catarina, Brazil.
Methods
Randomized clinical trial including obese pregnant women with a body mass index (BMI) ≥ 30 kg/m2, divided into two groups (control and metformin). Both groups received guidance regarding diet and physical exercise. The participants were assessed at two moments, the first at enrollment (gestational age ≤ 20) and the second at gestational weeks 24-28. The outcomes assessed were BMI and gestational diabetes mellitus (GDM) diagnosis. The data distribution was assessed with the Friedman test. For all the analytical models, the p-values were considered significant when lower than 0.05. The absolute risk reduction was also estimated.
Results
Overall, 164 pregnant women were assessed and further divided into 82 participants per group. No significant difference was observed in BMI variation between the control and metformin groups (0.9 ± 1.2 versus 1.0 ± 0.9, respectively, p = 0.63). Gestational diabetes mellitus was diagnosed in 15.9% (n = 13) of the patients allocated to the metformin group and 19.5% (n = 16) of those in the control group (p = 0.683). The absolute risk reduction was 3.6 (95% confidence interval 8.0- 15.32) in the group treated with metformin, which was not significant.
Conclusion
Metformin was not effective in reducing BMI and preventing GDM in obese pregnant women.
-
Review Article07-02-2008
Treatment of infertility in women with polycystic ovary syndrome
Revista Brasileira de Ginecologia e Obstetrícia. 2008;30(4):201-209
Abstract
Review ArticleTreatment of infertility in women with polycystic ovary syndrome
Revista Brasileira de Ginecologia e Obstetrícia. 2008;30(4):201-209
DOI 10.1590/S0100-72032008000400008
Views99See morePolycystic ovary syndrome (PCOS) occurs in 6 to 10% of women during the reproductive age. Insulin resistance and compensatory hyperinsulinemia are currently two of the main factors involved in the etiopathogenesis of PCOS. The objective of the present review was to discuss the controversies related to the treatment of infertile women with PCOS and during their pregnancy, focusing on the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) current consensus.


