Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2014;36(6):251-258
06-01-2014
DOI 10.1590/S0100-720320140004976
To assess the effects of a soy dietary supplement on the main biomarkers of cardiovascular health in postmenopausal women compared with the effects of low-dose hormone therapy (HT) and placebo.
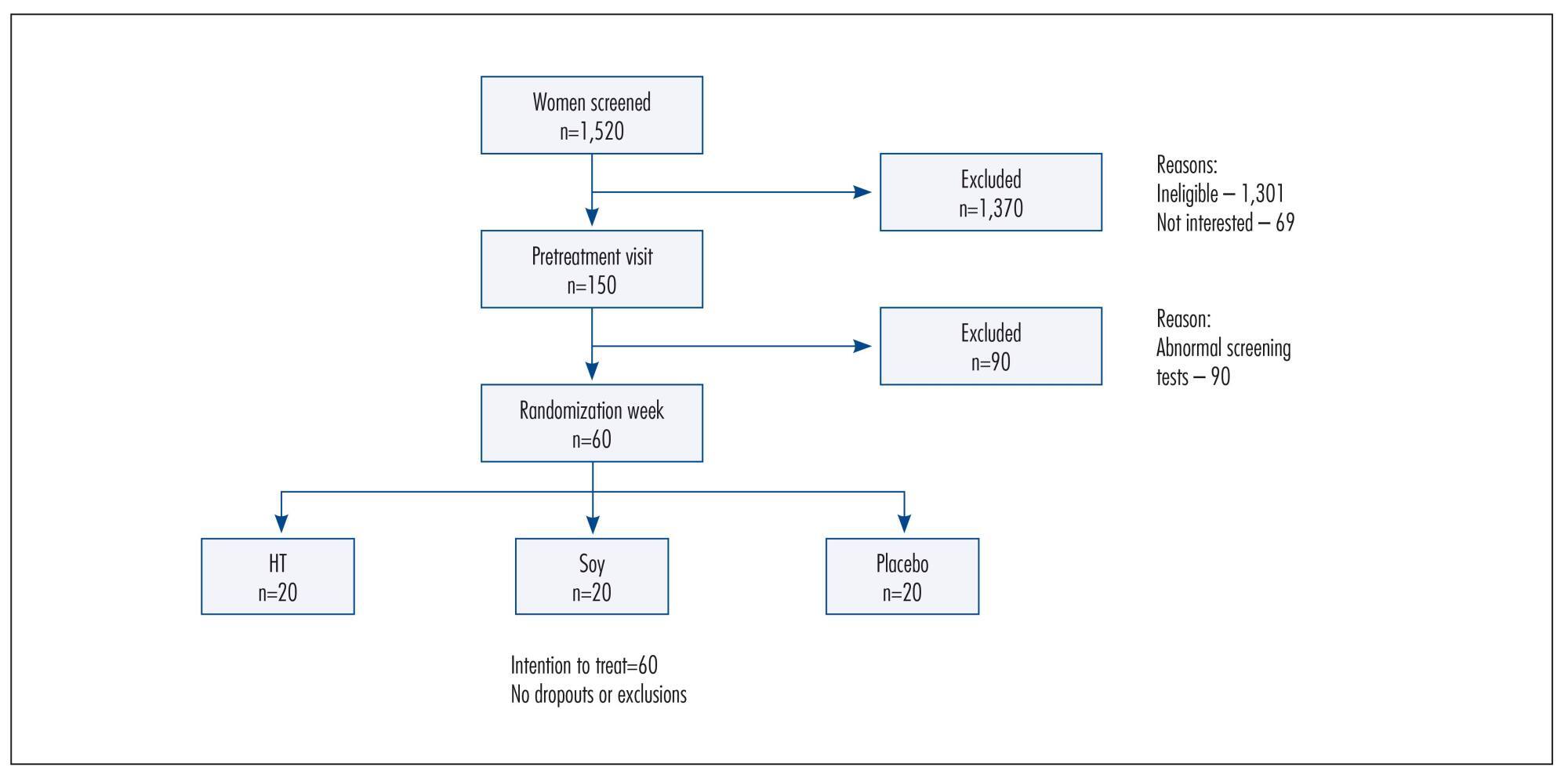
Double-blind, randomized and controlled intention-to-treat trial. Sixty healthy postmenopausal women, aged 40-60 years, 4.1 years mean time since menopause were recruited and randomly assigned to 3 groups: a soy dietary supplement group (isoflavone 90mg), a low-dose HT group (estradiol 1 mg plus noretisterone 0.5 mg) and a placebo group. Lipid profile, glucose level, body mass index, blood pressure and abdominal/hip ratio were evaluated in all the participants at baseline and after 16 weeks. Statistical analyses were performed using the χ2 test, Fisher's exact test, Kruskal-Wallis non-parametric test, analysis of variance (ANOVA), paired Student's t-test and Wilcoxon test.
After a 16-week intervention period, total cholesterol decreased 11.3% and LDL-cholesterol decreased 18.6% in the HT group, but both did not change in the soy dietary supplement and placebo groups. Values for triglycerides, HDL-cholesterol, glucose level, body mass index, blood pressure and abdominal/hip ratio did not change over time in any of the three groups.
The use of dietary soy supplement did not show any significant favorable effect on cardiovascular health biomarkers compared with HT. Clinical Trial Registry: The trial is registered at the Brazilian Clinical Trials Registry (Registro Brasileiro de Ensaios Clínicos - ReBEC), number RBR-76mm75.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 1998;20(5):273-280
04-12-1998
DOI 10.1590/S0100-72031998000500007
An open-label comparative study was conducted in nine centers in Brazil to evaluate the tolerability and cycle control of two low-dose oral contraceptives containing 20 mg ethynylestradiol/75 mg gestodene and 20 mg ethynylestradiol/150 mg desogestrel, during six treatment cycles. A total of 167 healthy sexually active women were enrolled (77 in the gestodene group and 90 in the desogestrel group) and 138 completed the six-cycle treatment period. A lipid and hemostatic profile was performed for a subgroup of first users. A total of 867 cycles were evaluated. Irregular bleeding did not occur in 95.4% of the cycles evaluated with gestodene and in 91.9% with desogestrel. Tolerability was good with both preparations but there was significantly more nausea in the desogestrel group. Cycle control was good with both preparations with a significantly lower incidence of irregular bleeding with gestodene when all cycles were considered. There were no clinically significant changes in the hemostatic profile. Lipid profile showed a trend to be more favorable after six cycles of treatment with both preparations. Women in the gestodene group did not present changes in the mean weight; in the desogestrel group there was a significant mean weight increase of 1 kg after six cycles of treatment. Compliance with treatment was good with both preparations. Results of this study demonstrated that low-dose preparations containing gestodene or desogestrel combined with 20 mg of ethynylestradiol are well-tolerated oral contraceptives that provide good cycle control.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2000;22(1):37-41
10-13-2000
DOI 10.1590/S0100-72032000000100007
Purpose: to evaluate the effects of tibolone on climacteric symptoms and clinical and metabolic variables. Methods: thirty-four postmenopausal women were treated orally with 2.5 mg tibolone daily for 48 weeks and evaluated as to climacteric complaints, clinical aspects such as weight and blood pressure and lipid profile (total cholesterol, HDL-c, LDL-c, VLDL-c and triglycerides). Results: a significant improvement of climacteric complaints was demonstrated by a significant decrease in the Kupperman index (p<0.001) and the mean number of hot flushes (p<0.001) from the first month of treatment onwards. There was a significant decrease in total cholesterol, triglycerides and VLDL-c (p<0.001). The LDL-c levels presented a slight decrease (not significant). The HDL-c levels showed a significant decrease at week 24. However these levels returned to baseline levels at week 48. With regard to the vital signs no change in body weight and blood pressure was measured. The side effects were mild and temporary, vaginal bleeding, nausea and edema being the most common. Conclusion: tibolone may be considered a safe and efficient option to treat climacteric symptoms in postmenopausal women without significant impact on lipid profile.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2004;26(7):573-578
10-14-2004
DOI 10.1590/S0100-72032004000700010
PURPOSE: to evaluate the effects of raloxifene on plasma homocysteine concentration and lipid profile in postmenopausal women. METHODS: twenty-four healthy postmenopausal women, aged 50 to 70 years, with osteopenia and/or osteoporosis, were submitted to raloxifene therapy, 60 mg/day, for six months. Plasma homocysteine concentration was determined before and after three and six months of therapy, as well as total cholesterol, HDL-cholesterol LDL-cholesterol and triglyceride levels. Plasma homocysteine was measured by a polarized immunofluorescence assay and serum lipids by the enzymatic and colorimetric method. Data were analyzed statistically by ANOVA, Newman-Keuls test and Pearson's correlation test. RESULTS: a significant decrease in total cholesterol of 15.3% (227.6±56.3 vs 200.6±29.8 vs 192.8±32.1 mg/dl; p<0.001) and LDL-cholesterol of 21.4% (151.4±46.3 vs 122.7±29.4 vs 119.0±28.6 mg/dl; p<0.001), and a significant increase in HDL-cholesterol of 9.5% (44.7±10.8 vs 52.2±12.6 vs 49.0±10.8 mg/dl; p<0.05) were observed. There was no reduction in triglyceride levels (134.9±50.3 vs 127.5±50.0 vs 121.0±36.0 mg/dl; p>0.05). Although not significant, a decrease in homocysteine by 4.5% (11.7±3.0 vs 11.0±2.9 vs 11.2±2.1 muM/l; p>0.05) was observed between the pre-and posttreatment periods, with a significant negative correlation between basal levels and posttreatment percentual reduction (r=-0.71; p<0.0001). CONCLUSIONS: raloxifene treatment, 60 mg/day, for six months caused a significant decrease in total and LDL-cholesterol and an increase in HDL-cholesterol in postmenopausal women. Plasma homocysteine concentration tended to decrease, this effect being more favorable in patients with elevated baseline levels.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2003;25(5):337-343
09-08-2003
DOI 10.1590/S0100-72032003000500006
PURPOSE: to evaluate the effects of soy germ isoflavone on menopausal symptoms and blood lipids in postmenopausal women. METHODS: a prospective study was performed on 50 women, randomly divided into two groups: 25 women on soy germ isoflavones (60 mg/day, capsules) (G1) and 25 women on placebo (G2). Inclusion criteria: women with hot flushes and FSH >40 mIU/mL, non-vegetarian, non-smoker, non-Asiatic, not in use of hormone replacement therapy and without disease of the gastrointestinal tract. For six months, the menopausal Kupperman index (MKI) and hormonal and lipid profiles were assessed. For statistical analysis, ANOVA, t test and the non-parametric Kruskal-Wallis and Mann-Whitney tests were used. RESULTS: the median MKI values, initially similar in both groups, decreased in G1 at two and four months (MKI = 14 and 9, respectively), and in G2 at two months (MKI = 15) (p<0.01). At six months, isoflavone was significantly superior to placebo in reducing hot flushes (44 versus 12%, respectively). At the end of the study, in the isoflavone group, an increase in estradiol levels (from 16,8±7.3 to 18,0±6.7 ng/dL) (p<0.05) was observed, with no alteration in FSH, LH and vaginal cytology; there was also a reduction of 11.8% in LDL (from 151.5±39.2 for 133,6±26.4 mg/dL) and a HDL increase of 27.3% (from 44.0±11.3 to 56.0±11.9 mg/dL) (p<0.05). CONCLUSIONS: soy germ isoflavone induced favorable effects on menopausal symptoms and lipid profile, showing to be an interesting option for alternative therapy in postmenopausal women.