Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo85
10-23-2024
To evaluate the role of being human immunodeficiency virus (HIV) positive for predicting the risk of recurrence in women with a cervical high grade squamous intraepithelial lesion (HSIL) diagnosis.
Retrospective observational case-control study, comprising HIV positive (case) and HIV negative (control) women in a 1:4 ratio. Women assisted by the Erasto Gaertner Hospital, between 2009-2018, with cervical HSIL diagnosis, submitted to treatment by Loop electrosurgical excision procedure (LEEP), and with a minimum follow-up of 18 months, were included. The immunological status, number and time to recurrence were analyzed, with p<0.05 considered significant. In a second analysis, only patients with free margins were evaluated.
The sample consisted of 320 women (64 cases and 256 controls). Presence of HIV, CD4 levels <200 and detectable viral load (CV) were associated with high risk of recurrence, with odds ratio (OR) of 5.4 (p<0.001/95CI:2.8-10); 3.6 (p<0.001 /IC95:0.6-21.1) and 1.8 (p=0.039 /IC95:0.3-9.3), respectively. In the sample with free margins (n=271), this risk was also higher among seropositive patients, with OR 4.18 (p=0.001/95CI:1.8-9.2).
HIV is an independent risk factor for cervical HSIL recurrence and reduced disease-free survival time. Glandular involvement, compromised margins, undetectable CV and CD4<200 also increase the risk of relapse.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(5):231-238
05-01-2016
To evaluate the incidence and factors associated with cervical intraepithelial neoplasia (CIN) and cervical infection by human papillomavirus (HPV) among HIV-positive and HIV-negative women.
A cohort of 103 HIV positive and 113 HIV negative women were monitored between October 2008 and February 2012, for at least one year. Procedures included cervical cytology, DNA/HPV detection by polymerase chain reaction, colposcopy with biopsy if necessary, followed by an interview for exposure characteristics data. CIN was based on the histopathological results.
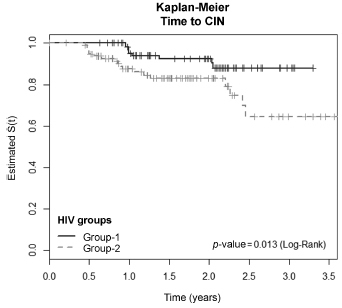
The incidence of CIN was of 8.8 and 4.6 cases/100 women-years in HIVpositive and HIV-negative women, respectively. HIV-positive women presented a hazard ratio (HR) of 2.8 for CIN and developed lesions earlier (0.86 year) than HIVnegative women (2 years) (p = 0.01). The risk of developing CIN decreased with age (HR = 0.9) and marital status (HR = 0.4). HPV patients presented a higher incidence of CIN when compared HIV-positive and HIV-negative women (p = 0.01). The incidence of HPV cervical infection was 18.1 and 11.4 cases/100 women-years in HIV-positive and HIV-negative women, respectively. Those HIV-positive presented earlier HPV infection (p = 0.002). The risk of developing HPV infection decreased with age and was higher among HIV-positive women. HPV 16 was the most common type in HIV-positive women, and also the type most closely associated with CIN in HIV-negative women.
HIV-positive women had a greater incidence of HPV and CIN, and in a shorter time interval. More rigorous and timely clinical control is required for this group.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2015;37(9):421-427
09-01-2015
DOI 10.1590/SO100-720320150005355
To evaluate the prevalence of toxoplasmosis, rubella, cytomegalovirus, hepatitis B&C and syphilis (Torchs) in a cohort pregnant women and to identify the sociodemographic, clinical and laboratory factors.
A total of 1,573 HIV-infected pregnant women from a Brazilian metropolitan region were studied between 1998 and 2013. The results of serological tests were available for 704 (44.8%) pregnant women. Pregnant women were considered to be Torchs positive (Gtp) when they had positive results for at least one of these infections, and to be Torchs negative (Gtn) when they had negative results for all of them. Maternal covariables were: age, marital status, educational level, time and mode of infection, CD4 lymphocyte count, viral load at delivery, and use of antiretroviral therapy (ARV). Neonatal covariables were: HIV infection, prematurity, low birth weight, neonatal complications, abortion and neonatal death. Odds ratios with 95% confidence interval were used to quantify the association between maternal and neonatal variables and the presence of Torchs.
Among 704 pregnant women, 70 (9.9%; 95%CI 7.8-12.4) had positive serological tests for any Torchs factor. The individual prevalence rates were: 1.5% (10/685) for toxoplasmosis; 1.3% (8/618) for rubella; 1.3% (8/597) for cytomegalovirus; 0.9% (6/653) for hepatitis B and 3.7% (20/545) for hepatitis C; and 3.8% (25/664) for syphilis. The HIV Vertical HIV transmission was 4.6% among Gtp pregnant women and 1.2% among Gtn women. Antiretroviral therapy (ARV), vertical transmission, low birth weight and neonatal complications were significantly associated with Torchs positivity in univariate analysis.
The Torchs prevalence found in the study was high for some infections. These findings emphasize the need to promote serological Torchs screening for all pregnant women, especially HIV-infected women, so that an early diagnosis can be made and treatment interventions can be implemented to prevent vertical HIV transmission.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2014;36(12):555-561
12-01-2014
DOI 10.1590/So100-720320140005155
To determine if illicit drug use increases the vertical transmission of HIV, to identify the risk factors involved in mother and child health and the prevalence of illicit drug use among these pregnant women.
Sixty-four (7.6%) of 845 pregnant women from the metropolitan region of Belo Horizonte, Minas Gerais, Brazil, attended in the service between October 1997 and February 2012 reported the use of illicit drugs. Cases were HIV-positive drug users (n=64) and controls were women who did not use drugs (n=192). Three controls were selected for each case. Several conditions of exposure were considered in the control group such as tobacco use, alcohol use, alcohol and tobacco use, maternal age, educational level, ethnicity, and marital status. Problems during the prenatal period, delivery and postpartum, vertical HIV transmission and neonatal outcomes were also investigated.
Univariate analysis showed as significant variables: maternal age, tobacco use, number of prenatal care visits, antiretroviral therapy, mode of infection, and viral load at delivery. Logistic regression revealed as significant variables: maternal age (less than 25 years); tobacco use, and number of prenatal care visits (less than 6). The vertical transmission of HIV was 4,8% (95%CI 1.7–13.3) among drug users and 2,1% (95%CI 0.8–5.2) in the control group, with no statistically significant difference between groups. Neonatal complications were more frequent among drug users, but also with no statistically significant difference between groups.
The use of illicit drug is frequent during pregnancy among HIV-infected women. The approach to illicit drug use should be routine during prenatal care visits. These women are more discriminated against and tend to deny their habits or do not seek prenatal care. There was no difference in vertical virus transmission between groups, probably indicating adherence to antiretroviral use for antiretroviral therapies during pregnancy.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2010;32(4):184-190
07-02-2010
DOI 10.1590/S0100-72032010000400006
PURPOSE: to analyze the clinical and epidemiological profile, the outcome of pregnancy and the vertical transmission of human immunodeficiency virus (HIV)-infected pregnant women receiving prenatal care at the University Hospital of Santa Maria (HUSM). METHODS: A prospective study was conducted on 139 HIV-infected pregnant women attended at the High-Risk Prenatal Care Outpatient Clinic of HUSM, during the period from August 2002 to August 2007, with at least two prenatal visits in this service. Data were collected by an interview and by filling out a research protocol during a prenatal visit. The protocol was attached to the medical records of the patient and kept until the outcome of gestation. Descriptive analysis of quantitative variables was performed using the SPSS software, version 15.0. RESULTS: The mean age of the 139 pregnant women studied was 25.6 years (±5.8), 79 (56.8%) were white, 81 (58.5%) were married or lived in a stable union, and 90 (65.0%) had less than eight years of schooling. Fifty-one percent of the pregnant women already had two or more children, with a number of children higher than the mean for the state. The infection was diagnosed during the present or a previous pregnancy in more than 70.0% cases. Sexual exposure occurred in 97.0%, and in 59.6% of cases the partner was known to be infected. During the study period, among the cases properly monitored, only one newborn (0.7%) was infected with HIV. CONCLUSIONS: Young women in a socioeconomic situation of vulnerability, with low schooling and multiparous represent the majority of HIV-positive pregnant women attended at the service. Evaluations performed during the prenatal period were relevant for the diagnosis of infection in most cases. An early diagnosis associated with proper clinical, obstetrical and psychological monitoring and with nursing care is important to provide appropriate treatment compliance and a reduction of the rates of vertical transmission.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2008;30(9):437-444
10-23-2008
DOI 10.1590/S0100-72032008000900003
PURPOSE: to verify the accuracy of uterine cervix cytology for HPV diagnosis, as compared to polymerase chain reaction (PCR) in samples of women with HIV. METHODS: 158 patients who had undergone a first collection of material from the uterine cervix with Ayre's spatula for PCR were included in the study. Then, another collection with Ayre's spatula and brush for oncotic cytology was performed. Only 109 slides were reviewed, as 49 of them had already been destructed for have being filed for over two years. RESULTS: the prevalence of HPV was 11% in the cytological exam and 69.7% in the PCR. Age varied from 20 to 61 years old, median 35 years. The HIV contagious route was heterosexual in 91.8% of the cases, and 79.1% of the patients had had from one to five sexual partners along their lives. The most frequent complaint was pelvic mass (5.1%), and 75.3% of the women had looked for the service for a routine medical appointment. The categorical variable comparison was done through contingency tables, using the χ2 test with Yates's correction to compare the ratios. The Fisher's test was used when one of the expected rates was lower than five. In the comparison of diagnostic tests, sensitivity, specificity and similarity ratios have been calculated. Among the 76 patients with HPV, detected by PCR, only 12 had the diagnosis confirmed by cytology (sensitivity=15.8%), which on the other hand did not present any false-positive results (specificity=100%). Concerning the HPV presence, the cytological prediction for positive results was 100% and 33.3% for negative, when both results were compared. Among the 12 patients with HPV positive cytology, four (33.3%) presented cervical intraepithelial neoplasia (OR=56; positive similarity ratio=positive infinity; negative similarity ratio=0.83). CONCLUSIONS: As the cytology specificity is quite high, it is possible to rely on the positive result, which means that a positive result will surely indicate the presence of HPV. The low sensitivity of cytology does not qualify it as a survey exam for HPV detection in this female group.