-
Original Article
Improving Implantation Rate in 2nd ICSI Cycle through Ovarian Stimulation with FSH and LH in GNRH Antagonist Regimen
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(10):749-758
12-17-2021
Summary
Original ArticleImproving Implantation Rate in 2nd ICSI Cycle through Ovarian Stimulation with FSH and LH in GNRH Antagonist Regimen
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(10):749-758
12-17-2021Views138Abstract
Objective
To investigate whether patients with a previous recombinant follicle stimulating hormone (rFSH)-stimulated cycle would have improved outcomes with rFSH + recombinant luteinizing hormone (rLH) stimulation in the following cycle.
Methods
For the present retrospective case-control study, 228 cycles performed in 114 patients undergoing intracytoplasmic sperm injection (ICSI) between 2015 and 2018 in an in vitro fertilization (IVF) center were evaluated. Controlled ovarian stimulation (COS) was achieved with rFSH (Gonal-f, Serono, Geneva, Switzerland) in the first ICSI cycle (rFSH group), and with rFSH and rLH (Pergoveris, Merck Serono S.p.A, Bari, Italy) in the second cycle (rFSH + rLH group). The ICSI outcomes were compared among the groups.
Results
Higher estradiol levels, oocyte yield, day-3 high-quality embryos rate and implantation rate, and a lower miscarriage rate were observed in the rFSH + rLH group compared with the rFSH group. In patients < 35 years old, the implantation rate was higher in the rFSH + rLH group compared with the rFSH group. In patients ≥ 35 years old, higher estradiol levels, oocyte yield, day-3 high-quality embryos rate, and implantation rate were observed in the rFSH + rLH group. In patients with ≤ 4 retrieved oocytes, oocyte yield, mature oocytes rate, normal cleavage speed, implantation rate, and miscarriage rate were improved in the rFSH + rLH group. In patients with ≥ 5 retrieved oocytes, higher estradiol levels, oocyte yield, and implantation rate were observed in the rFSH + rLH group.
Conclusion
Ovarian stimulation with luteinizing hormone (LH) supplementation results in higher implantation rates, independent of maternal age and response to COS when compared with previous cycles stimulated with rFSH only. Improvements were also observed for ICSI outcomes and miscarriage after stratification by age and retrieved oocytes.
Key-words Follicle stimulating hormoneintracytoplasmic sperm injection implantationLuteinizing hormoneOvarian stimulationSee more -
Review Article
Morphology and Biochemistry of Ovulation Morfologia e bioquímica da ovulação
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(6):480-486
07-27-2021
Summary
Review ArticleMorphology and Biochemistry of Ovulation Morfologia e bioquímica da ovulação
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(6):480-486
07-27-2021Views181See moreAbstract
The process of ovulation involves multiple and iterrelated genetic, biochemical, and morphological events: cessation of the proliferation of granulosa cells, resumption of oocyte meiosis, expansion of cumulus cell-oocyte complexes, digestion of the follicle wall, and extrusion of the metaphase-II oocyte. The present narrative review examines these interrelated steps in detail. The combined or isolated roles of the folliclestimulating hormone (FSH) and luteinizing hormone (LH) are highlighted. Genes indiced by the FSH genes are relevant in the cumulus expansion, and LH-induced genes are critical for the resumption ofmeiosis and digestion of the follicle wall. A nonhuman model for follicle-wall digestion and oocyte release was provided.
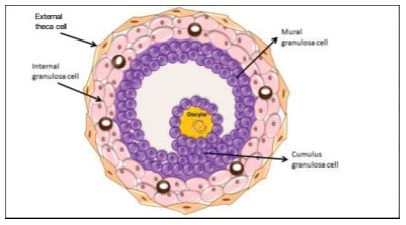
-
Artigos Originais
Correlation between age and antral follicles count in infertile women
Revista Brasileira de Ginecologia e Obstetrícia. 2012;34(4):184-188
05-11-2012
Summary
Artigos OriginaisCorrelation between age and antral follicles count in infertile women
Revista Brasileira de Ginecologia e Obstetrícia. 2012;34(4):184-188
05-11-2012DOI 10.1590/S0100-72032012000400008
Views97PURPOSE: To produce age-related nomograms for ovarian antral follicle count (AFC) in infertile women. METHODS: It was done a cross-sectional study of patients attended in the center of assisted reproduction Fêmina, from March 2010 to October 2011. The patients were submitted to transvaginal ultrasonography from day 2 to day 4 of their menstrual period. Patients included were between 21 to 45 years old, with regular menses, two healthy ovaries, without any evidence of endocrinopathies and who gave written informed consent. Patients excluded were smokers, with galactosemia or ovarian cysts, with antecedents of liver disease, ovarian surgeries or who were treated with chemotherapy or radiotherapy. In order to check the evolution of the AFC in relation to patient age, we used the 5th, 25th, 50th, 75th and 95th percentiles. Linear regression was carried out using these percentiles, permitting us to determine the effect of age on the CFA. RESULTS: A total of 172 patients with a mean age of 32.7 years were included in the trial. The male and tubal factors were the main causes of infertility, accounting for 65% of cases. The age-related nomogram for the 5th, 25th, 50th, 75th and 95th percentiles of AFC revealed that changes were best fitted by a linear function. The percentiles that showed the highest correlations were 25 (r=-0.9; p<0.001), 50 (r=-0.9; p<0.001) and 75 (r=-0.9; p<0.001). CONCLUSION: A nomogram was constructed correlating age with the different AFC percentiles in infertile women without endocrinopathies. This showed a linear pattern of decline in AFC with age in all percentiles. These nomograms could provide a reference guide for the clinician. However, future validation, with longitudinal data, still is needed.
Key-words Age factorsfemaleFollicle stimulating hormoneInfertilityNomogramsOvarian follicleUltrasonographySee more -
Artigos Originais
Evaluation of basal FSH serum levels in infertile patients with deep ovarian endometriosis who underwent surgery
Revista Brasileira de Ginecologia e Obstetrícia. 2009;31(7):349-352
10-09-2009
Summary
Artigos OriginaisEvaluation of basal FSH serum levels in infertile patients with deep ovarian endometriosis who underwent surgery
Revista Brasileira de Ginecologia e Obstetrícia. 2009;31(7):349-352
10-09-2009DOI 10.1590/S0100-72032009000700005
Views79See morePURPOSE: to evaluate the ovarian reserve of infertile patients with severe ovarian endrometriosis, submitted to excisional surgery of endometriomas and attended from February to November, 2008. METHODS: prospective study, including 30 patients with endometriosis grades III and IV, with severe ovarian impairment, submitted to excisional surgery of the endometriomas, and 30 patients with endometriosis grades I and II, allocated as a Control Group. The ovarian reserve was indirectly assessed, through the basal (U/L) follicle stimulating hormone (FSH), between the third and fifth days of the cycle, 12 months after the surgery. The body mass index (BMI) was calculated according to Quetelet's formula [weight (kg)/height(cm²)]. The Mann-Whitney non-parametric U test was used to compare the variables "age", "BMI" and "basal SFH" between the groups. RESULTS: there was no significant difference between the groups about age and BMI. Concerning basal FSH, in the group of patients with severe endometriosis, the average value was 7.0 U/L, while in the Control Group, it was 5.6 U/L (p=0.3), what demonstrates that the difference between the two groups was not significant. CONCLUSIONS: the surgery did not affect the ovarian reserve of patients with severe ovarian endometriosis.
-
Artigos Originais
Evidence of follicle responsiveness to FSH by antimüllerian hormone in ovulating women
Revista Brasileira de Ginecologia e Obstetrícia. 2009;31(3):142-147
06-08-2009
Summary
Artigos OriginaisEvidence of follicle responsiveness to FSH by antimüllerian hormone in ovulating women
Revista Brasileira de Ginecologia e Obstetrícia. 2009;31(3):142-147
06-08-2009DOI 10.1590/S0100-72032009000300007
Views49See morePURPOSE: to test the hypothesis that the anti-müllerian hormone (AMH) serum level reflects the antral follicles' response to the administration of FSH. METHODS: prospective study, including 116 normo-ovulatory infertile patients submitted to controlled ovarian hyperstimulation with GnRH and FSH agonists. The AMH serum level was measured after reaching the pituitary suppression and before the FSH administration (basal day). The number of antral follicles was determined by ultrasonography at the basal day (precocious antral follicles; 2 to 8 mm) and at the day of hCG administration (dhCG; pre-ovulatory follicles; >16 mm). The follicle response to FSH was determined by the percentage of precocious antral follicles which reached pre-ovulatory stage in response to FSH (maturation rate). The correlation of AMH with the patients' age, the total number of precocious antral and pre-ovulatory follicles, collected oocytes, total dose of FHS in the controlled ovarian stimulation and the rate of follicular maturation was studied. For the statistical analysis, it simple regression analysis and the Spearman's test were used, at a 5% significance level. RESULTS: The serum level of AMH was positively correlated with the number of precocious antral follicles at the basal day (r=0.64; p<0.0001) and pre-ovulatory follicles in dhCG (r=0.23; p=0.01). Exceptionally, the serum level of AMH was negatively correlated with the maturation ratio (r=-0.24; p<0.008). CONCLUSIONS: AMH attenuates the follicular development caused by FSH administration.
-
Artigos Originais
Relationship of serum anti-Müllerian hormone, inhibin B, estradiol and FSH on day 3 with ovarian follicular status
Revista Brasileira de Ginecologia e Obstetrícia. 2007;29(4):186-191
07-30-2007
Summary
Artigos OriginaisRelationship of serum anti-Müllerian hormone, inhibin B, estradiol and FSH on day 3 with ovarian follicular status
Revista Brasileira de Ginecologia e Obstetrícia. 2007;29(4):186-191
07-30-2007DOI 10.1590/S0100-72032007000400004
Views50See morePURPOSE: to examine the hypothesis that serum anti-Müllerian hormone (AMH) levels reflect the ovarian follicular status. METHODS: Design: prospective study. Patients: we studied 101 IVF-ET candidates undergoing controlled ovarian hyperstimulation with GnRH agonist and FSH. After the achievement of pituitary suppression and before FSH administration (baseline), serum AMH, inhibin B, and FSH levels were measured. The number of antral follicles was determined by ultrasound at baseline (early antral follicles; 3-10 mm). RESULTS: at baseline, median serum levels of AMH, inhibin B, E2, P4 and FSH were 3.42±0.14 ng/mL, 89±4.8 pg/mL, 34±2.7 pg/mL, 0.22±0.23 ng/mL and 6.6±0.1 mIU/mL, respectively, and the mean number of early antral follicles was 17±0.39. Serum levels of AMH were negatively correlated with age (r=-0.19, p<0.04), and positively correlated with number of antral follicles (r=0.65, p<0.0001), but this did not apply to serum levels of either inhibin B, E2 or FSH. CONCLUSION: the data demonstrate an association between AMH and antral follicular counts. Therefore, AMH is probable a biomarker of ovarian follicular status.
-
Artigo de Revisão
Gonadotropin level changes during the reproductive life
Revista Brasileira de Ginecologia e Obstetrícia. 2007;29(1):48-55
04-04-2007
Summary
Artigo de RevisãoGonadotropin level changes during the reproductive life
Revista Brasileira de Ginecologia e Obstetrícia. 2007;29(1):48-55
04-04-2007DOI 10.1590/S0100-72032007000100008
Views97See moreChanges in the levels of gonadotropins throughout the reproductive life depend on a fine tuned functional development of neural pathways and GnRH-neurones, pituitary gonadotrophs and granulosa-theca cells of the follicular wall. Both, LH and FSH levels change according to the day-time, menstrual cycle phase, and gynecological age. Initiating the puberty, changes in LH pulses are remarkable, showing higher frequency and amplitude at night. Later in puberty, the pulses of LH are also maintained during the day, remaining its levels with very little variation within the 24 hours period. During the menstrual cycle, the FSH levels increase at the end of the luteal phase, decrease during the medium and late follicular phase, increase rapidly in the ovulatory phase and remain at low basal levels until the late luteal phase. The levels of LH remain unaltered during the whole follicular phase, increase in the ovulatory surge, and decrease to the basal levels in the luteal phase. At the forth decade of life, the GnRH secretion changes, with hypothalamic loss of sensitivy to the estradiol positive feedback and decrease in frequency and prolongation of the GnRH pulses. The pituitary response is atenuated due to decrease in the density of GnRH receptors on gonadotroph cells, loss of gonadotroph sensitivity, secretion of more basic FSH and LH molecules, decrease in frequency and increase in amplitude of LH and FSH pulses. These modifications result in monotropic increase of the FSH secretion. Current studies show that the selective increase in the FSH levels in the early follicular phase is gradual, beginning as early as the third decade of life. These alterations in FSH are associated with an accelerated follicular depletion in women after 37-38 years old. On the other side, the LH levels remain almost constant up to the end of reproductive life. The different levels of FSH and LH seen throughout the reproductive years may be due to yet unknown regulatory mechanisms in the hypothalamic-pituitary-ovarian axis.
-
Artigos Originais
FSH, LH, estradiol, progesterone, and histamine concentrations in serum, peritoneal fluid and follicular fluid of women with and without endometriosis
Revista Brasileira de Ginecologia e Obstetrícia. 2006;28(11):643-651
02-12-2006
Summary
Artigos OriginaisFSH, LH, estradiol, progesterone, and histamine concentrations in serum, peritoneal fluid and follicular fluid of women with and without endometriosis
Revista Brasileira de Ginecologia e Obstetrícia. 2006;28(11):643-651
02-12-2006DOI 10.1590/S0100-72032006001100003
Views97PURPOSE: literature reports show that there are no conclusive data about the association between endometriosis and the concentrations of hormones involved in the control of reproduction. Thus, the present study was undertaken to determine FSH, LH, estradiol (E), progesterone (P), and histamine (Hi) concentrations in serum, peritoneal fluid and follicular fluid of women with and without endometriosis. METHODS: the extent of the disease was staged according to the revised American Fertility Society classification (1997). For the collection of serum and peritoneal fluid, 28 women with endometriosis undergoing diagnostic laparoscopy were selected (18 infertile women with endometriosis I-II and ten infertile women with endometriosis III-IV). For the control group, 21 fertile women undergoing laparoscopy for tubal sterilization were selected. Follicular fluid was obtained from 39 infertile women undergoing in vitro fertilization (21 women with endometriosis and 18 women without endometriosis). RESULTS: FSH and LH levels in serum, peritoneal fluid and follicular fluid did not differ significantly between groups. On the other hand, E and P concentrations in the peritoneal fluid were significantly lower in infertile women with endometriosis (E: 154.2±15.3 for stages I-II and 89.3 ng/mL±9.8 ng/mL for stages III-IV; P: 11.2±1.5 for stages I-II and 7.6 ng/mL±0.8 for stages III-IV) in comparison with control women (E: 289.1 ng/mL±30.1; P: 32.8±4.1 ng/mL) (Kruskal-Wallis/Dunn tests; p<0.05). In serum, estradiol and progesterone concentrations followed the same pattern. In the follicular fluid, E and Hi concentrations were significantly lower in women with endometriosis (E: 97.4±11.1 pg/mL; Hi: 6.6±0.9 ng/mL) in comparison to women without endometriosis (E: 237.5±28.5 pg/mL; Hi: 13.8±1.3 ng/mL) (Student t-test; p<0.05), while progesterone levels revealed no significant difference between groups. CONCLUSIONS: our results indicate ovary dysfunction in women with endometriosis, with reduction on E, P and Hi concentrations, which may contribute to the subfertility often associated with the disease.
Key-words EndometriosisEstradiolFertilization in vitroFollicle stimulating hormoneHistamineInfertility, femaleLuteinizing hormoneProgesteroneSee more


