-
Original Article07-10-2023
Maternal Blood Fatty Acid Levels in Fetal Growth Restriction
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(3):127-133
Abstract
Original ArticleMaternal Blood Fatty Acid Levels in Fetal Growth Restriction
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(3):127-133
Views247See moreAbstract
Objective:
To assess the maternal blood levels of fatty acids (FAs) in pregnancies with fetal growth restriction (FGR).
Methods:
This prospective cross-sectional study included pregnant women with gestational age between 26 and 37 + 6 weeks with FGR and appropriate for gestational age (AGA) fetuses. The levels of saturated, trans, monounsaturated, and polyunsaturated FAs were measured using centrifugation and liquid chromatography. The Student’s t-test, Mann-Whitney test, and general linear model, with gestational age and maternal weight as covariants, were used to compare FA levels and the FGR and AGA groups. The Chi-square was used to evaluate the association between groups and studied variables.
Results:
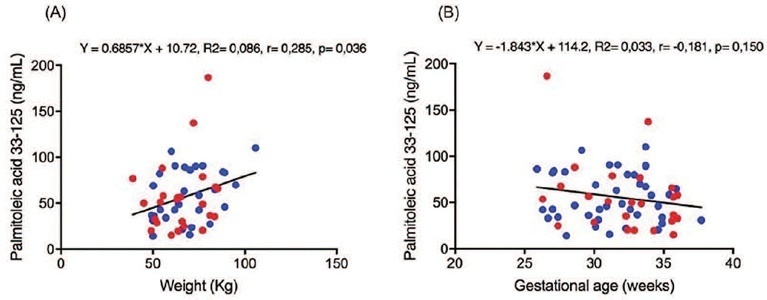
Maternal blood sample was collected from 64 pregnant women, being 24 FGR and 40 AGA. A weak positive correlation was found between the palmitoleic acid level and maternal weight (r = 0.285, p = 0.036). A weak negative correlation was found between the gamma-linoleic acid level and gestational age (r = −0.277, p = 0.026). The median of the elaidic acid level (2.3 vs. 4.7ng/ml, p = 0.045) and gamma-linoleic acid (6.3 vs. 6.6ng/ml, p = 0.024) was significantly lower in the FGR than the AGA group. The palmitoleic acid level was significantly higher in the FGR than AGA group (50.5 vs. 47.6ng/ml, p = 0.033).
Conclusion:
Pregnant women with FGR had lower elaidic acid and gamma-linoleic acid levels and higher palmitoleic acid levels than AGA fetuses.
-
Review Article07-10-2023
Comparison between Protocols for Management of Fetal Growth Restriction
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(2):096-103
Abstract
Review ArticleComparison between Protocols for Management of Fetal Growth Restriction
Revista Brasileira de Ginecologia e Obstetrícia. 2023;45(2):096-103
Views241See moreAbstract
This comprehensive review compares clinical protocols of important entities regarding the management of fetal growth restriction (FGR), published since 2015. Five protocols were chosen for data extraction. There were no relevant differences regarding the diagnosis and classification of FGR between the protocols. In general, all protocols suggest that the assessment of fetal vitality must be performed in a multimodally, associating biophysical parameters (such as cardiotocography and fetal biophysical profile) with the Doppler velocimetry parameters of the umbilical artery, middle cerebral artery, and ductus venosus. All protocols reinforce that the more severe the fetal condition, the more frequent this assessment should be made. The timely gestational age and mode of delivery to terminate the pregnancy in these cases can vary much between the protocols. Therefore, this paper presents, in a didactic way, the particularities of different protocols for monitoring FGR, in order to help obstetricians to better manage the cases.
-
Original Article04-08-2022
Analysis of the Correlation/Agreement of Maternal-fetal Doppler Parameters in Normal and Growth-Restricted Fetuses
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(2):118-124
Abstract
Original ArticleAnalysis of the Correlation/Agreement of Maternal-fetal Doppler Parameters in Normal and Growth-Restricted Fetuses
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(2):118-124
Views191See moreAbstract
Objective
To assess the degree of correlation/agreement of maternal-fetal Doppler parameters between normal and growth-restricted fetuses (fetal growth restriction [FGR]).
Methods
The present observational and retrospective study included 274 singleton pregnancies. The following maternal-fetal Doppler parameters were assessed: uterine artery (UAt), umbilical artery (UA), middle cerebral artery (MCA), cerebroplacental ratio (CPR), and umbilical-cerebral ratio (U/C). The assessment of FGR was based on the Figueiras and Gratacós9 criteria. Spearman correlation coefficients were estimated to assess the correlation between resistance (RI) and pulsatility (PI) indices of Doppler parameters. The agreement between two Doppler parameters was assessed by the Kappa coefficient.
Results
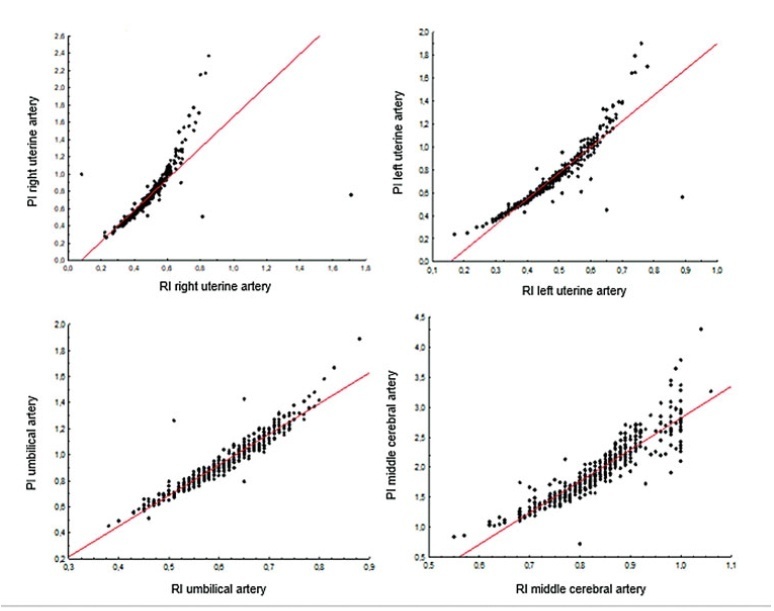
In total, 502 Doppler examinations were included, and FGR was observed in 19 out of 274 fetuses. A strong correlation was observed between RI and PI of UAt, UA, and MCA in all of the samples (p<0.001). Of the 502 Doppler examinations, there was agreement between U/C and CPR percentiles for 480 (95.6%) and disagreement for 22 (4.4%), with Kappa coefficient of 0.26, thereby corresponding to weak agreement. Of the 68 cases with estimated fetal weight ≤ 9th percentile (small for gestational age [SGA]), there was agreement between U/C>1.0 and CPR<5th percentile in 61 (88.4%) and disagreement in 7 (5.8%) with Kappa coefficient of 0.49, thereby corresponding to moderate agreement.
Conclusion
Strong correlation was observed among RI and PI UAt, UA, and MCA Doppler examinations in the present study; however, weak agreement was observed between U/C and CPR in the normal and FGR fetuses. In SGA, U/C and CPR demonstrated moderate agreement.
-
Original Article02-17-2022
The Role of High Concentrations of Homocysteine for the Development of Fetal Growth Restriction
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(4):352-359
Abstract
Original ArticleThe Role of High Concentrations of Homocysteine for the Development of Fetal Growth Restriction
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(4):352-359
Views169Abstract
Objective
To assess homocysteine (Hcy) levels in the three trimesters of pregnancy in women with fetal growth restriction (FGR) and to evaluate the role of Hcy as a possible predictor of FGR.
Methods
A total of 315 singleton pregnant women were included in the present prospective cohort study and were monitored since the 1st trimester of pregnancy before delivery. Newborns were monitored for the first 7 days of life. Patients who had risk factors for FGR were excluded. Fetal growth restriction was defined according to uterine fundal height (< 10 percentile), ultrasound fetometry (< 5 percentile), and anthropometry of newborns (<5 percentile). The concentrations of Hcy were detected at between 10 and 14, between 20 and 24, and between 30 and 34 weeks of pregnancy by enzyme-linked immunosorbent assay (ELISA). Receiver operating characteristics (ROC) curve test and diagnostic odds ratio (DOR) were performed to evaluate the results of ELISA.
Results
The concentration of Hcy in patients with FGR was 19.65 umol/L at between 10 and 14 weeks, compared with 9.28 umol/L in patients with normal fetal growth (p<0.0001). The optimal cut-off level for Hcy in the 1st trimester of pregnancy was>13.9 umol/L with AUC 0.788, sensitivity of 75%, specificity of 83.6%, and DOR of 15.2.
Conclusion
Assessment of serum Hcy concentration may be used as a predictor of FGR, with the highest diagnostic utility in the 1st trimester of pregnancy.
Key-words Fetal growth restrictionHomocysteinehyperhomocysteinemiaprediction of fetal growth restrictionSee more -
Original Article12-17-2021
The Assessment of Vitamin D Levels in Pregnant Women is not Associated to Fetal Growth Restriction: A Cross Sectional Study
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(10):743-748
Abstract
Original ArticleThe Assessment of Vitamin D Levels in Pregnant Women is not Associated to Fetal Growth Restriction: A Cross Sectional Study
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(10):743-748
Views247See moreAbstract
Objective
To assess maternal serum levels of vitamin D in fetuses appropriate for gestational age (AGA), small for gestational age (SGA), and with fetal growth restriction (FGR) according to estimated fetal weight (EFW).
Methods
This cross-sectional study included 87 pregnant women between 26 and 36 weeks of gestation: 38 in the AGA group, 24 in the SGA group, and 25 in the FGR group. Maternal serum vitamin D levels were assessed using the chemiluminescence method. The Fisher exact test was used to compare the results between the groups.
Results
The mean ± standard deviation (SD) of maternal age (years) and body mass index (kg/m2) in the AGA, SGA, and FGR groups were 25.26 8.40 / 26.57 ± 4.37; 25.04 ± 8.44 / 26.09 ± 3.94; and 25.48 ± 7.52 / 26.24 ± 4.66, respectively (p > 0.05). The maternal serum vitamin D levels (mean ± SD) of the AGA, SGA, and FGR groups were 22.47 ± 8.35 ng/mL, 24.80 ± 10.76 ng/mL, and 23.61 ± 9.98 ng/mL, respectively, but without significant differences between the groups (p = 0.672).
Conclusion
Maternal serum vitamin D levels did not present significant differences among pregnant women with AGA, SGA, or FGR fetuses between 26 and 36 weeks of gestation according to EFW.
-
Review Article10-18-2021
Prenatal Ultrasound Diagnosis of Biometric changes in the Brain of Growth Restricted Fetuses. A Systematic Review of Literature
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(7):545-559
Abstract
Review ArticlePrenatal Ultrasound Diagnosis of Biometric changes in the Brain of Growth Restricted Fetuses. A Systematic Review of Literature
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(7):545-559
Views153See moreAbstract
Fetal growth restriction (FGR) occurswhen the fetus does not reach its intrauterine potential for growth and development as a result of compromise in placental function. It is a condition that affects 5 to 10% of pregnancies and is the second most common cause of perinatal morbidity and mortality. Children born with FGR are at risk of impaired neurological and cognitive development and cardiovascular or endocrine diseases in adulthood. The purpose of the present revision is to perform a literature search for evidence on the detection and assessment by ultrasound of brain injury linked to FGR during fetal life. Using a systematic approach and quantitative evaluation as study methodology, we reviewed ultrasound studies of the fetal brain structure of growth-restricted fetuses with objective quality measures. A total of eight studies were identified. High quality studies were identified for measurement of brain volumes; corpus callosum; brain fissure depth measurements, and cavum septi pellucidi width measurement. A low-quality study was available for transverse cerebellar diameter measurement in FGR. Further prospective randomized studies are needed to understand the changes that occur in the brain of fetuseswith restricted growth, as well as their correlation with the changes in cognitive development observed.
-
Original Article02-03-2019
Perinatal Outcomes of Fetuses with Early Growth Restriction, Late Growth Restriction, Small for Gestational Age, and Adequate for Gestational Age
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(12):688-696
Abstract
Original ArticlePerinatal Outcomes of Fetuses with Early Growth Restriction, Late Growth Restriction, Small for Gestational Age, and Adequate for Gestational Age
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(12):688-696
Views229See moreAbstract
Objective
To evaluate the association between early-onset fetal growth restriction (FGR), late-onset FGR, small for gestational age (SGA) and adequate for gestational age (AGA) fetuses and adverse perinatal outcomes.
Methods
This was a retrospective longitudinal study in which 4 groups were evaluated: 1 - early-onset FGR (before 32 weeks) (n=20), 2 - late-onset FGR (at or after 32 weeks) (n=113), 3 - SGA (n=59), 4 - AGA (n=476). The Kaplan-Meier curve was used to compare the time from the diagnosis of FGR to birth. Logistic regression was used to determine the best predictors of adverse perinatal outcomes in fetuses with FGR and SGA.
Results
A longer timebetween the diagnosis and birthwas observed forAGAthan for late FGR fetuses (p<0.001). The model including the type of FGR and the gestational age at birth was significant in predicting the risk of hospitalization in the neonatal intensive care unit (ICU) (p<0.001). The model including only the type of FGR predicted the risk of needing neonatal resuscitation (p<0.001), of respiratory distress (p<0.001), and of birth at<32, 34, and 37 weeks of gestation, respectively (p<0.001).
Conclusion
Fetal growth restriction and SGA were associated with adverse perinatal outcomes. The type of FGR at the moment of diagnosis was an independent variable to predict respiratory distress and the need for neonatal resuscitation. The model including both the type of FGR and the gestational age at birth predicted the risk of needing neonatal ICU hospitalization.


