Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(12):615-622
12-01-2016
Breast cancer is the most common type of cancer and the leading cause of cancer-related death among women worldwide. Hormone receptor-positive (HRþ) tumors represent the most common form of this disease, with more than 70% of breast cancers expressing these receptors. Response and benefit to neoadjuvant chemo-therapy (NCT) varies according to HR expression, with lower responses in luminal tumors as compared with hormone receptor-negative (HR-) and human epidermal growth factor receptor 2-positive (HER2þ) tumors. Neoadjuvant endocrine therapy (NET) is an option for selected patients with HRþ locally advanced breast cancer. Neoadjuvant endocrine therapy has a favorable toxicity profile, and is associated with benefits such as having low cost and being more easily available even for cancer care professionals outside major urban areas or tertiary centers. These factors are particularly relevant, as 70% of breast cancer deaths occur in women from low-income and middle-income countries. Additionally, NET is being increasingly explored, not simply to allow for less extensive surgery, but also as a scientific tool, with the use of biomarkers to predict outcomes in adjuvant trials and for the individual patient. This review details the current and most relevant evidence about NET for breast cancer as well as the future directions of this field.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2015;37(6):283-290
06-01-2015
DOI 10.1590/SO100-720320150005292
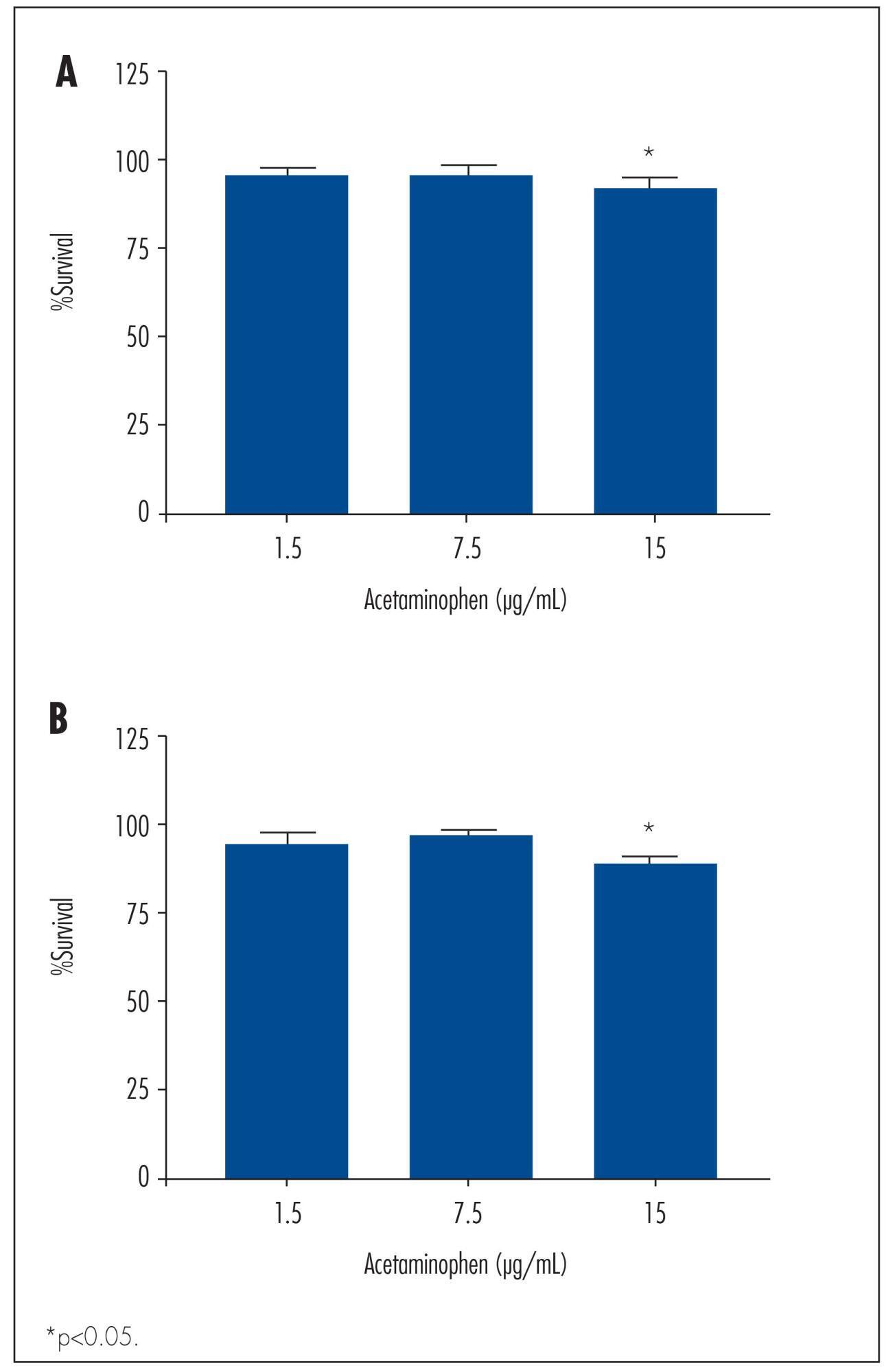
To determine the basic expression of ABC transporters in an epithelial ovarian cancer cell line, and to investigate whether low concentrations of acetaminophen and ibuprofen inhibited the growth of this cell line in vitro.
TOV-21 G cells were exposed to different concentrations of acetaminophen (1.5 to 15 μg/mL) and ibuprofen (2.0 to 20 μg/mL) for 24 to 48 hours. The cellular growth was assessed using a cell viability assay. Cellular morphology was determined by fluorescence microscopy. The gene expression profile of ABC transporters was determined by assessing a panel including 42 genes of the ABC transporter superfamily.
We observed a significant decrease in TOV-21 G cell growth after exposure to 15 μg/mL of acetaminophen for 24 (p=0.02) and 48 hours (p=0.01), or to 20 μg/mL of ibuprofen for 48 hours (p=0.04). Assessing the morphology of TOV-21 G cells did not reveal evidence of extensive apoptosis. TOV-21 G cells had a reduced expression of the genes ABCA1, ABCC3, ABCC4, ABCD3, ABCD4 and ABCE1 within the ABC transporter superfamily.
This study provides in vitro evidence of inhibitory effects of growth in therapeutic concentrations of acetaminophen and ibuprofen on TOV-21 G cells. Additionally, TOV-21 G cells presented a reduced expression of the ABCA1, ABCC3, ABCC4, ABCD3, ABCD4 and ABCE1 transporters.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2005;27(6):331-339
11-11-2005
DOI 10.1590/S0100-72032005000600007
PURPOSE: to evaluate the epidemiologic data and signs of trophoblastic hyperplasia in patients with complete hydatidiform mole (CHM) and to estimate the risk associated with the persistence of the disease. METHODS:: we evaluated 214 patients with CHM submitted to uterine evacuation between 1980 and 2001. The patients were included prospectively. All patients were followed until negative bHCG with weekly clinical evaluation and bHCG quantification. We considered persistence when the patient needed another treatment after uterine evacuation. The risk factors for persistence were evaluated through univariate and multivariate analysis, and the odds ratio (OR) was calculated for each one. RESULTS: among the epidemiologic factors, only negative Rh was significant (OR=2.28). All signs of trophoblastic hyperplasia, represented by uterine size larger than expected, sonographic uterine volume, tecaluteinic cysts, and betaHCG higher than 10(5) were associated with risk for the presistence of the disease. The presence of at least one sign of trophoblastic hyperplasia showed sensitivity of 82% and predictive positive value of 35.1% (OR=4.8). The logistic regression identified larger uterine size than expected and bHCG higher than 10(5) as risk factors for persistence of the gestational trophoblastic disease (OR=4.1 and 5.5, respectively). CONCLUSIONS: the signs of trophoblastic hyperplasia showed good sensitivity to predict persistence of the disease; however, the low predictive positive value does not allow using these criteria to change treatment. It is very important to reinforce the importance of serial betaHCG quantification in these high-risk patients.