Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo69
To compare the effectiveness and safety of non-mRNA versus mRNA COVID-19 vaccines on pregnant women and their newborns in a systematic review with meta-analysis.
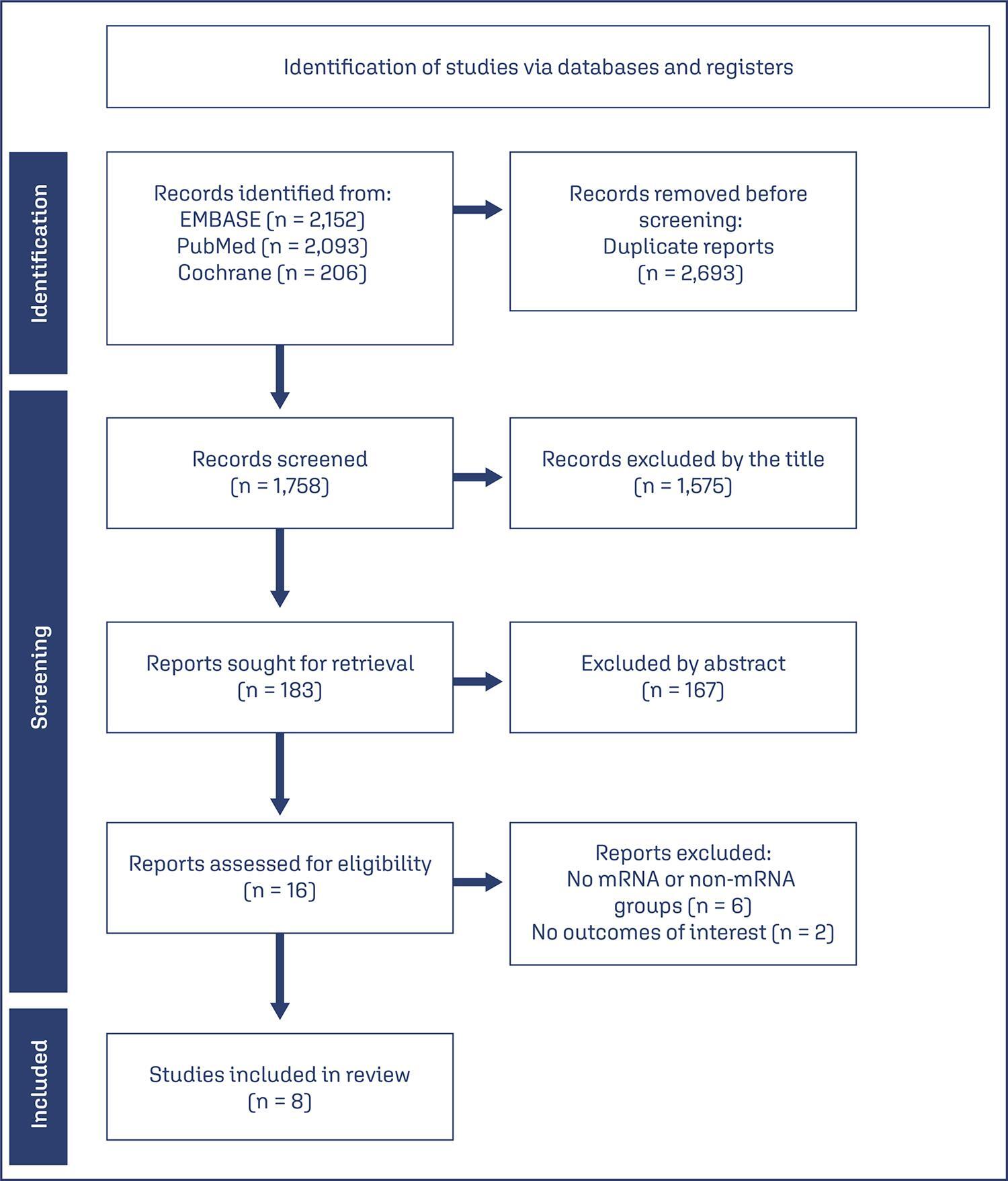
We searched PubMed, Embase, and Cochrane Central in May 2023.
The search strategy yielded 4451 results, 16 studies were fully reviewed. We selected case-control studies analysing non-mRNA versus mRNA vaccines. Data collection and analysis: we assessed the risk of bias using the Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool. Standardised mean differences were pooled using random-effect models.
We identified 8 prospective and retrospective studies with a total of 32,153 patients. Non-mRNA vaccines were associated with a higher incidence of fever (OR 2.67; 95% CI 2.08-3.43; p<0.001), and a lower incidence of fetal or neonatal death (OR 0.16; 95% CI 0.08-0.33; p<0.001). In subgroup analyses, the Jansen vaccine (Ad26.COV2.S) was found to have a higher rate of premature labor/delivery (OR 4.48; 95% CI 1.45-13.83; p=0.009) and missed/spontaneous abortion (OR 1.90; 95% CI 1.09-3.30; p=0.02), as compared with the Pfizer (BNT162b2) vaccine.
non-mRNA vaccines are associated with a lower incidence of fetal or neonatal death among pregnant women who receive a Covid19 vaccine, although at an increased rate of pyrexia compared with mRNA vaccines. Other studies are required for better assessment.
CRD42023421814
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2022;44(9):821-829
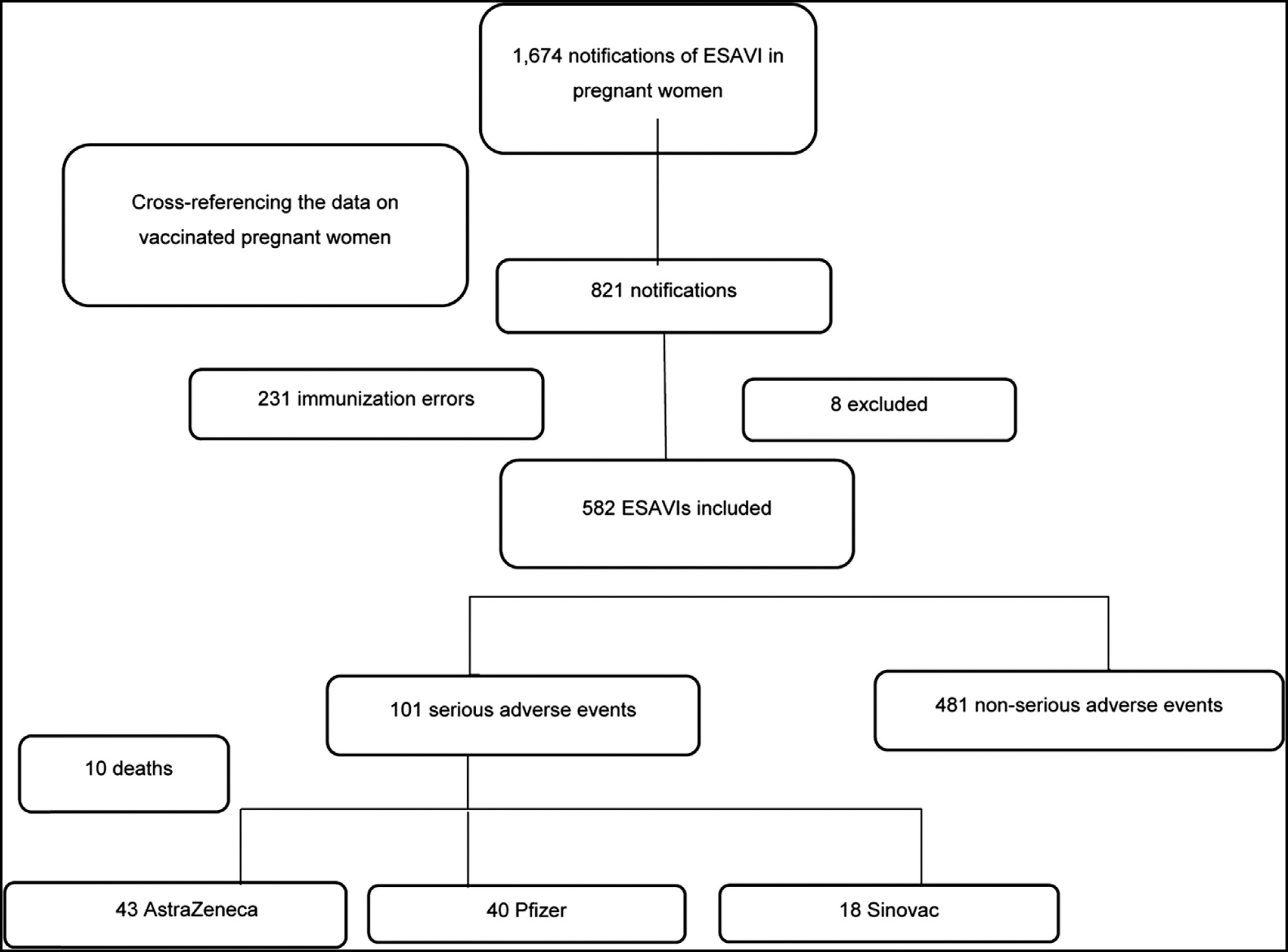
Regulations for the vaccination of pregnant women in Brazil occurred in March 2021. Despite the absence of robust data in the literature on the coronavirus disease 2019 (COVID-19) vaccinations in pregnant women, it is understood that the benefit-risk ratio tends to be favorable when considering the pandemic and the high burden of the disease. However, it is still important to monitor for Events Supposedly Attributable to Vaccination or Immunization (ESAVI) and to draw safety profiles of the different platforms used in pregnant and postpartum women. The present study aims to describe the main characteristics of ESAVIs related to COVID-19 vaccines occurring in pregnant women in the first months of the vaccination campaign in Brazil. During the evaluation period, 1,674 notifications of ESAVIs in pregnant women were recorded, and 582 notifications were included for the analysis. Of the 582 ESAVIs identified, 481 (82%) were classified as non-serious adverse events and 101 (17%) as serious adverse events. Ten deaths were identified, including one death which was considered to be causally related to the vaccine. The other nine maternal deaths had causality C, that is, without causal relationship with the vaccine, and most were due to complications inherent to pregnancy, such as pregnancy-specific hypertensive disorder (PSHD) in 4 cases and 3 due to COVID-19. Despite some limitations in our study, we believe it brings new insights into COVID-19 vaccines in this group and will add to the available evidence.