-
Original Article
Comparison between Enzyme Immunoassays Performed on Samples of Dried Blood and Serum for Toxoplasmosis Prenatal Screening: Population-based Study
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(5):351-356
07-30-2021
Summary
Original ArticleComparison between Enzyme Immunoassays Performed on Samples of Dried Blood and Serum for Toxoplasmosis Prenatal Screening: Population-based Study
Revista Brasileira de Ginecologia e Obstetrícia. 2021;43(5):351-356
07-30-2021Views170Abstract
Objective
Most prenatal screening programs for toxoplasmosis use immunoassays in serum samples of pregnant women. Few studies assess the accuracy of screening tests in dried blood spots, which are of easy collection, storage, and transportation. The goals of the present study are to determine the performance and evaluate the agreement between an immunoassay of dried blood spots and a reference test in the serum of pregnant women from a population-based prenatal screening program for toxoplasmosis in Brazil.
Methods
A cross-sectional study was performed to compare the immunoassays Imunoscreen Toxoplasmose IgM and Imunoscreen Toxoplasmose IgG (Mbiolog Diagnósticos, Ltda., Contagem, Minas Gerais, Brazil)in dried blood spots with the enzymelinked fluorescent assay (ELFA, BioMérieux S.A., Lyon, France) reference standard in the serum of pregnant women from Minas Gerais Congenital Toxoplasmosis Control Program.
Results
The dried blood spot test was able to discriminate positive and negative results of pregnant women when comparedwith the reference test, with an accuracy of 98.2% for immunoglobulin G (IgG), and of 95.8% for immunoglobulin M (IgM).
Conclusion
Dried blood samples are easy to collect, store, and transport, and they have a good performance,making this a promisingmethod for prenatal toxoplasmosis screening programs in countries with continental dimensions, limited resources, and a high prevalence of toxoplasmosis, as is the case of Brazil.
Key-words Congenital toxoplasmosisdried blood spot testingPrenatal careprenatal diagnosisToxoplasmosisSee more -
Original Article
Follow-up of Toxoplasmosis during Pregnancy: Ten-Year Experience in a University Hospital in Southern Brazil
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(9):539-547
09-30-2019
Summary
Original ArticleFollow-up of Toxoplasmosis during Pregnancy: Ten-Year Experience in a University Hospital in Southern Brazil
Revista Brasileira de Ginecologia e Obstetrícia. 2019;41(9):539-547
09-30-2019Views191See moreAbstract
Objective
To describe a population of pregnant women diagnosed with toxoplasmosis and their respective newborns, describing the hospital protocol for treatment and follow-up.
Methods
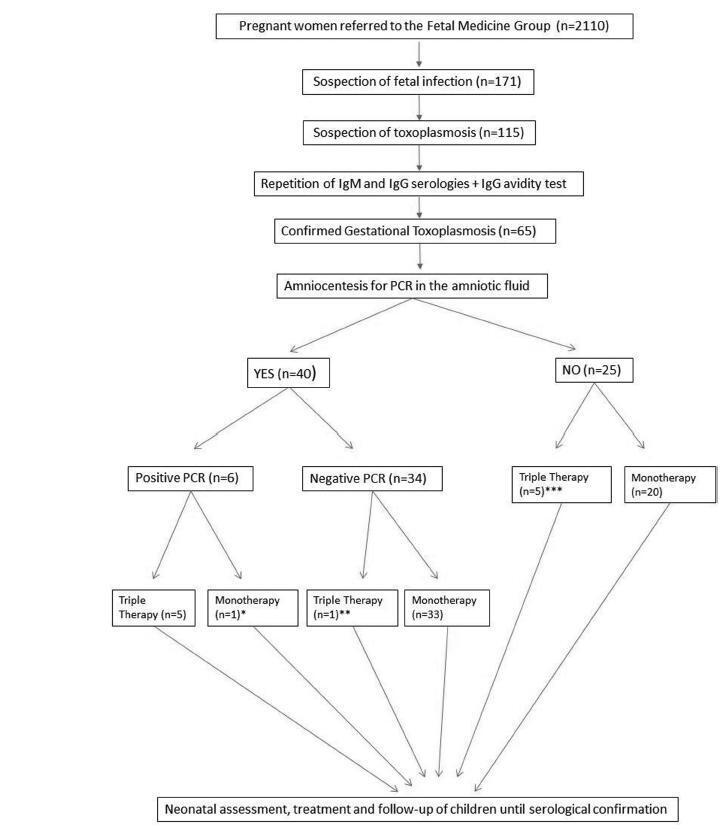
Retrospective cohort of pregnant women with acute toxoplasmosis infection and risk of transplacental transmission who were sent to the Fetal Medicine Group of Hospital de Clínicas de Porto Alegre (HCPA) between - January 1, 2006 and December 31, 2016. All patients with confirmed disease were included. The diagnostic protocol and treatment were applied; a polymerase chain reaction (PCR) analysis of the amniotic fluid was used to diagnose toxoplasmosis and determine the treatment. The newborns were followed up at the pediatric outpatient clinic specializing in congenital infection. The patients who were not followed up or were not born in the HCPA were excluded.
Results
A total of 65 patients were confirmed to have gestational toxoplasmosis; 40 performed amniocentesis, and 6 (15%) were identified as having positive PCR in the amniotic fluid. In five of those cases, this result associated with the gestational age defined the triple therapy during pregnancy, and in one case, it defined the monotherapy (advanced gestational age). A total of 4 of these newborns were treated from birth with triple therapy for 10months, 1 was not treated (due to maternal refusal), and 1 progressed to death within the first 54 hours of life due to complications of congenital toxoplasmosis. Of the 34 remaining cases with a negative PCR, 33 were treated with monotherapy and 1 was treated with triple therapy (ultrasound findings); of these children, 9 (26.5%) presented negative immunoglobulin G (IgG), 24 (70.6%) presented positive IgG (but none presented positive immunoglobulin M [IgM]), and 1 (2,9%) presented alterations compatible with congenital disease and started treatment with the triple therapy soon after birth. Out of the total sample of 60 patients, among the 25 who did not perform amniotic fluid PCR, 5 were treated with triple therapy (ultrasound findings/prior treatment) and 20 patients were submitted to monotherapy; only two newborns underwent treatment for congenital toxoplasmosis. Among the 65 cases of gestational toxoplasmosis, 6 (9,2%) children had a diagnosis of congenital toxoplasmosis, and 2 patients with triple therapy felt severe adverse effects of the medications.
Conclusions
The present study suggests that research on PCR screening of the amniotic fluid may be useful to identify patients with a higher potential for fetal complications, who may benefit from the poly-antimicrobial treatment. Patients with negative PCR results must continue to prevent fetal infection with monotherapy, without risk of fetal or maternal impairment.
-
Trabalhos Originais
Ultrasonographic markers for fetal congenital toxoplasmosis
Revista Brasileira de Ginecologia e Obstetrícia. 2004;26(5):377-382
08-04-2004
Summary
Trabalhos OriginaisUltrasonographic markers for fetal congenital toxoplasmosis
Revista Brasileira de Ginecologia e Obstetrícia. 2004;26(5):377-382
08-04-2004DOI 10.1590/S0100-72032004000500006
Views95See moreOBJECTIVE: to describe ultrasonographic alterations in fetuses infected with Toxoplasma gondii, correlating them with neonatal prognosis. METHODS: between June 1997 and May 2003, 150 pregnant women with suspected toxiplasmosis were examined. Acute infection was confirmed in 72 (48%) of these pregnant women and congenital toxoplasmosis was diagnosed in 12 (16%) fetuses. Prenatal diagnosis was established by polymerase chain reaction in the amniotic fluid. All the patients received antiparasitic therapy. Ultrasound examination was performed every fortnight and all the infants were evaluated during their first year of life. RESULTS: ultrasonographic changes were observed in eight fetuses. All of them showed symmetric bilateral ventricular enlargement that was associated with periventricular calcifications in five cases. Other changes as hepatic calcification, hepatomegaly, polyhydramnium, and pericardial effusion were less frequent. Among these fetuses, four were stillborn and three showed sequelae (chorioretinitis and neuro-psychomotor retardation). The four fetuses that showed normal ultrasonography had a satisfactory development. CONCLUSION: There was a high incidence of ultrasonographic changes in fetuses with congenital toxoplasmosis, mainly brain damage. Other changes as hepatomegaly and pericardial effusion were less frequent and were related to a systemic infection. The prognosis of these fetuses seems to be correlated with the presence of these lesions mainly because they had high mortality ratio and among the survivors the incidence of sequelae was high. The non-symptomatic fetuses evolved in a favorable way without developing sequelae. These results highlight the value of ultrasonographic examination of these fetuses in order to establish a prognosis and allow the elaboration of a suitable post-natal procedure.
-
Trabalhos Originais
A Comparison between Methods for the Diagnosis of Congenital Toxoplasmosis
Revista Brasileira de Ginecologia e Obstetrícia. 2001;23(5):277-282
06-26-2001
Summary
Trabalhos OriginaisA Comparison between Methods for the Diagnosis of Congenital Toxoplasmosis
Revista Brasileira de Ginecologia e Obstetrícia. 2001;23(5):277-282
06-26-2001DOI 10.1590/S0100-72032001000500002
Views123See moreObjective: to test the effectiveness of the polymerase chain reaction (PCR) in the amniotic fluid for the detection of fetal contamination due to Toxoplasma gondii in pregnant women with acute infection and to correlate it with the inoculation technique and the histology of the placenta. Methods: thirty-seven patients were prospectively studied and the diagnosis was based on the identification of maternal acute infection followed by amniocentesis guided by ultrasound to obtain amniotic fluid for PCR and mice inoculation. The mothers were treated with spiramycin throughout pregnancy; when fetal infection was demonstrated, pyrimethamine and sulfadiazine were added to the regimen. The placentas were processed for histologic examination. The infants were followed for a period that varied from three to 23 months for the confirmation or exclusion of congenital toxoplasmosis. Results: association measures such as sensitivity, specificity and predictive values were calculated for PCR in the amniotic fluid, detection of the parasite through mice inoculation and placental histology and showed the following results: PCR values of sensitivity = 66.7% and specificity = 87.1%; the respective values for mice inoculation were 50 and 100% and for the placental histology were 80 and 66.7%. Conclusion: although PCR should not be used alone for the prenatal diagnosis of congenital toxoplasmosis, it is a promising method and deserves more studies to improve its efficacy.


