-
Original Article06-01-2015
Gene expression profile of ABC transporters and cytotoxic effect of ibuprofen and acetaminophen in an epithelial ovarian cancer cell line in vitro
Revista Brasileira de Ginecologia e Obstetrícia. 2015;37(6):283-290
Abstract
Original ArticleGene expression profile of ABC transporters and cytotoxic effect of ibuprofen and acetaminophen in an epithelial ovarian cancer cell line in vitro
Revista Brasileira de Ginecologia e Obstetrícia. 2015;37(6):283-290
DOI 10.1590/SO100-720320150005292
Views142PURPOSES:
To determine the basic expression of ABC transporters in an epithelial ovarian cancer cell line, and to investigate whether low concentrations of acetaminophen and ibuprofen inhibited the growth of this cell line in vitro.
METHODS:
TOV-21 G cells were exposed to different concentrations of acetaminophen (1.5 to 15 μg/mL) and ibuprofen (2.0 to 20 μg/mL) for 24 to 48 hours. The cellular growth was assessed using a cell viability assay. Cellular morphology was determined by fluorescence microscopy. The gene expression profile of ABC transporters was determined by assessing a panel including 42 genes of the ABC transporter superfamily.
RESULTS:
We observed a significant decrease in TOV-21 G cell growth after exposure to 15 μg/mL of acetaminophen for 24 (p=0.02) and 48 hours (p=0.01), or to 20 μg/mL of ibuprofen for 48 hours (p=0.04). Assessing the morphology of TOV-21 G cells did not reveal evidence of extensive apoptosis. TOV-21 G cells had a reduced expression of the genes ABCA1, ABCC3, ABCC4, ABCD3, ABCD4 and ABCE1 within the ABC transporter superfamily.
CONCLUSIONS:
This study provides in vitro evidence of inhibitory effects of growth in therapeutic concentrations of acetaminophen and ibuprofen on TOV-21 G cells. Additionally, TOV-21 G cells presented a reduced expression of the ABCA1, ABCC3, ABCC4, ABCD3, ABCD4 and ABCE1 transporters.
Key-words AcetaminophenApoptosisATP-binding cassette transportersCell proliferationChemopreventionDrug therapyIbuprofenOvarian neoplasmsSee more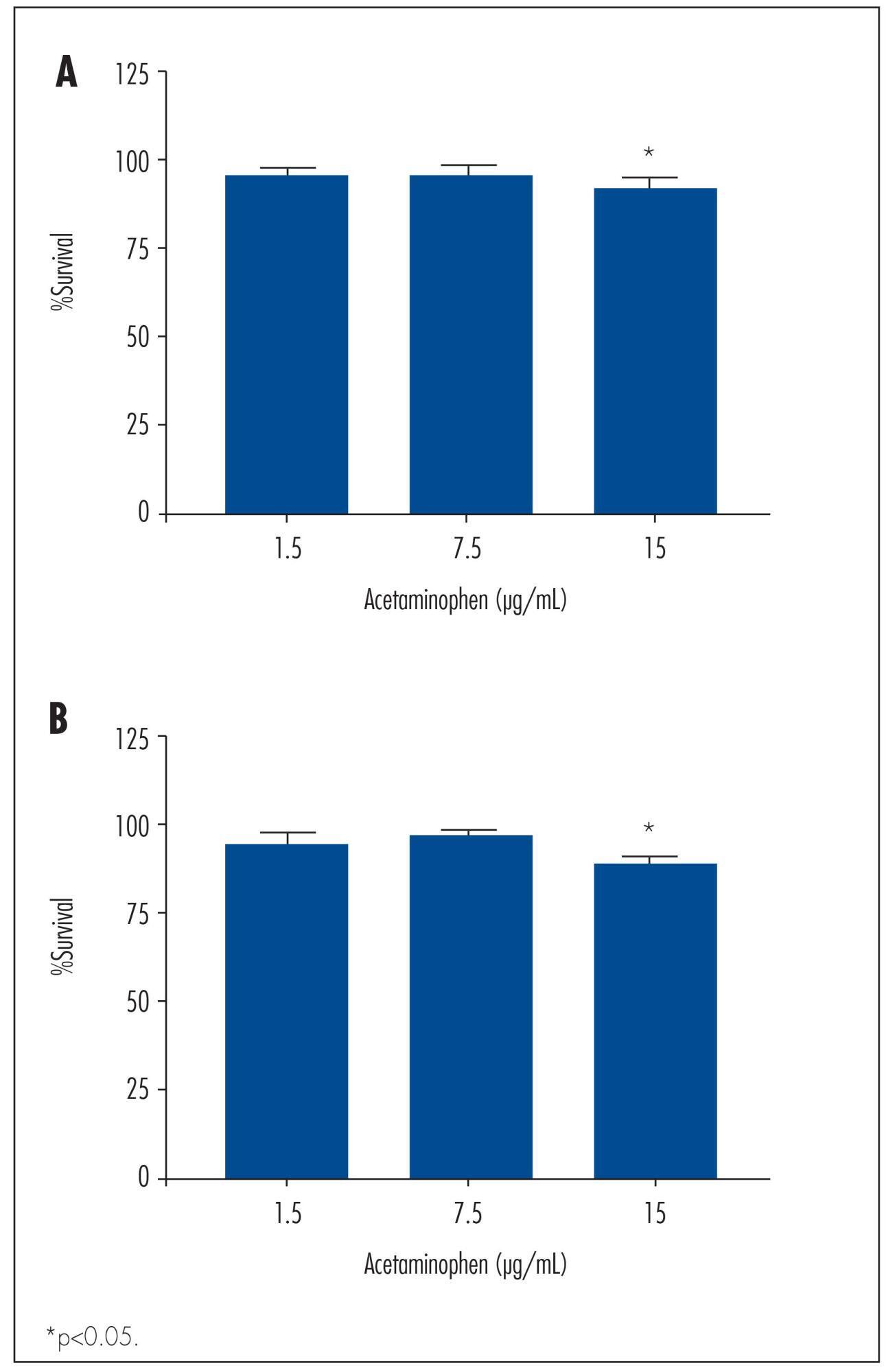
-
Original Article03-20-1999
Effects of the Chronic Use of Acetaminophen on Pregnant Rats
Revista Brasileira de Ginecologia e Obstetrícia. 1999;21(2):105-108
Abstract
Original ArticleEffects of the Chronic Use of Acetaminophen on Pregnant Rats
Revista Brasileira de Ginecologia e Obstetrícia. 1999;21(2):105-108
DOI 10.1590/S0100-72031999000200008
Views118See morePurpose: to evaluate the effects of acetaminophen on the pregnancy of female albino rats. Methods: forty pregnant rats were separated into four groups. All the animals received daily by gavage 1 ml of acetaminophen solution from the first day (day zero) until the 20th day of pregnancy: group I - only distilled water (control); groups II, III and IV, respectively, 125, 500 and 1,500 mg/kg body weight of acetaminophen dissolved in distilled water. The animals were weighed on days 0, 7, 15 and 20 of pregnancy. Results: our results showed that the rats that received the medication presented a reduction in weight when compared to the control group. The incidence of reabsorption of the embryos was 2.0, 3.5 and 7.0 times higher than in the control, in groups II, III and IV, respectively. Groups GII and GIV showed a clear reduction in the weight of the concepts. In GIV there was a 50% reduction in weight increase of fetuses and placentas when compared to the control, and 15.7% of external malformations were also found. Conclusions: the continuous use of acetaminophen should be avoided at doses higher than 70 mg/kg per during pregnancy.


