Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo9
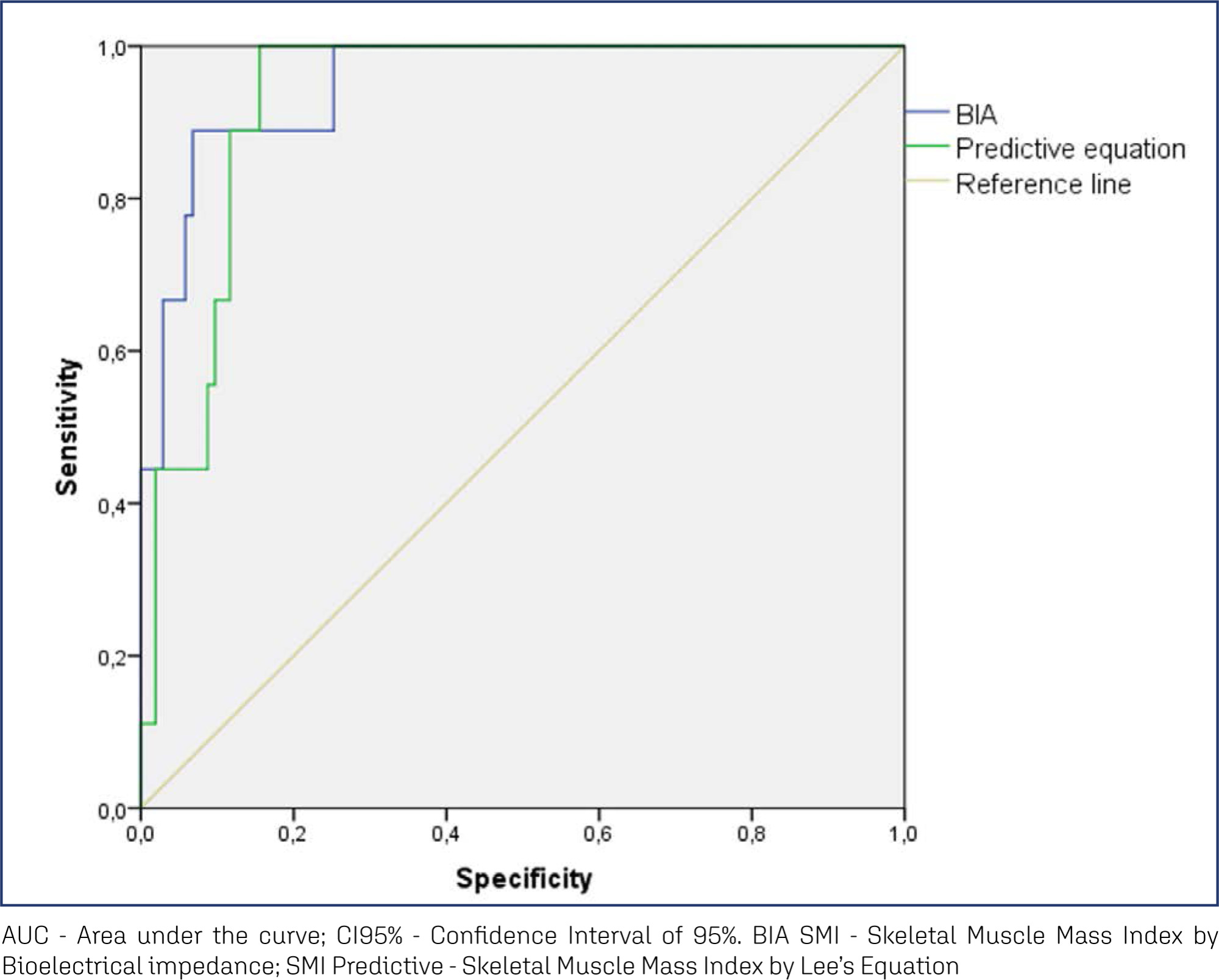
To analyze the amount of muscle and the presence of sarcopenia in postmenopausal women using different methods, verifying the agreement between them as to skeletal muscle mass (SMM).
This cross-sectional observational study was conducted with postmenopausal women aged ≥ 50 years. SMM was obtained from a predictive equation, Bioelectrical Impedance (BIA), and Dual Energy X-Ray Absorptiometry (DXA). The skeletal muscle mass index (SMI) and the appendicular skeletal muscle mass index (ASMI) were calculated. The cut-off point of SMI was determined for the population itself. The agreement between the SMI obtained using the different methods was verified. Sarcopenia was diagnosed according to the criteria proposed by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2). The significance level adopted for all tests was 5.0%.
A total of 112 women were evaluated, with an average age of 66.1 ± 5.65 years. Among them, 51.8% were sufficiently active and 43.8% were overweight and obese. The SMI cut-offs were 6.46 kg/m2 for the predictive equation and 7.66 kg/m2 for BIA, with high sensitivity and specificity. There was an excellent agreement in the identification of SMM by the predictive equation (0.89 [0.824-0.917], p < 0.001) and BIA (0.92 [0.883-0.945], p < 0.001), in reference to DXA. The prevalence of sarcopenia was 0.9%, 1.8%, and 2.7% according to BIA, DXA, and the predictive equation, respectively.
The predictive equation showed the expected agreement in estimating skeletal muscle mass in postmenopausal women, offering a viable and accurate alternative.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgoedt1
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgofps1
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgoedt2
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo56
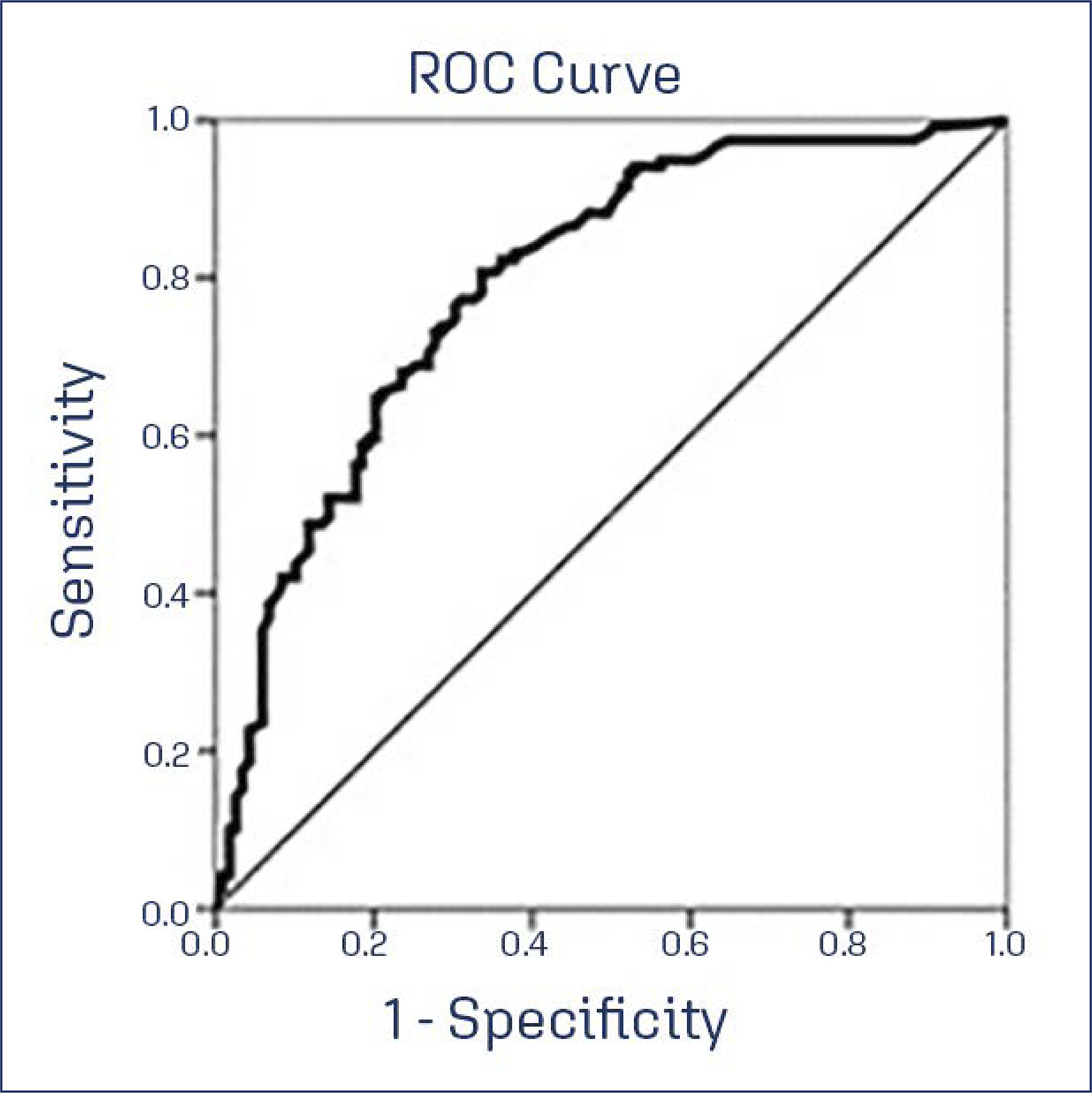
This study aimed to translate and validate the Estro-Androgenic-Symptom Questionnaire in Women (EASQ-W) into Brazilian Portuguese language, as we hypothesized that this tool would be consistent for addressing the specific context of hormonal symptoms in menopause.
In a cross-sectional study, a total of 119 women with Genitourinary Syndrome of Menopause (GSM) and 119 climacteric women without GSM were included. The EASQ-W was translated, and its psychometric properties were rigorously examined. Participants completed questionnaires covering sociodemographic details, the EASQ-W, and the Menopause Rating Scale (MRS). A subgroup of 173 women was re-invited after 4 weeks for test-retest analysis of the EASQ-W. Additionally, the responsiveness of the questionnaire was evaluated in 30 women who underwent oral hormonal treatment.
The internal consistency of the EASQ-W was found to be satisfactory in both GSM and control groups (Cronbach’s alpha ≥ 0.70). Notably, a floor effect was observed in both groups; however, a ceiling effect was only evident in the sexual domain of the GSM group. Construct validity was established by comparing the EASQ-W with the MRS, yielding statistically significant correlations (0.33831-0.64580, p < 0.001). The test-retest reliability over a 4-week period was demonstrated to be satisfactory in both the GSM and control groups (ICC 0.787-0.977). Furthermore, the EASQ-W exhibited appropriate responsiveness to oral hormonal treatment (p < 0.001).
This study successfully translated and validated the Estro-Androgenic-Symptom Questionnaire in Women (EASQ-W) into Brazilian Portuguese, with satisfactory internal consistency, test-retest reliability, and construct validity.
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-FPS07
Summary
Revista Brasileira de Ginecologia e Obstetrícia. 2024;46:e-rbgo29
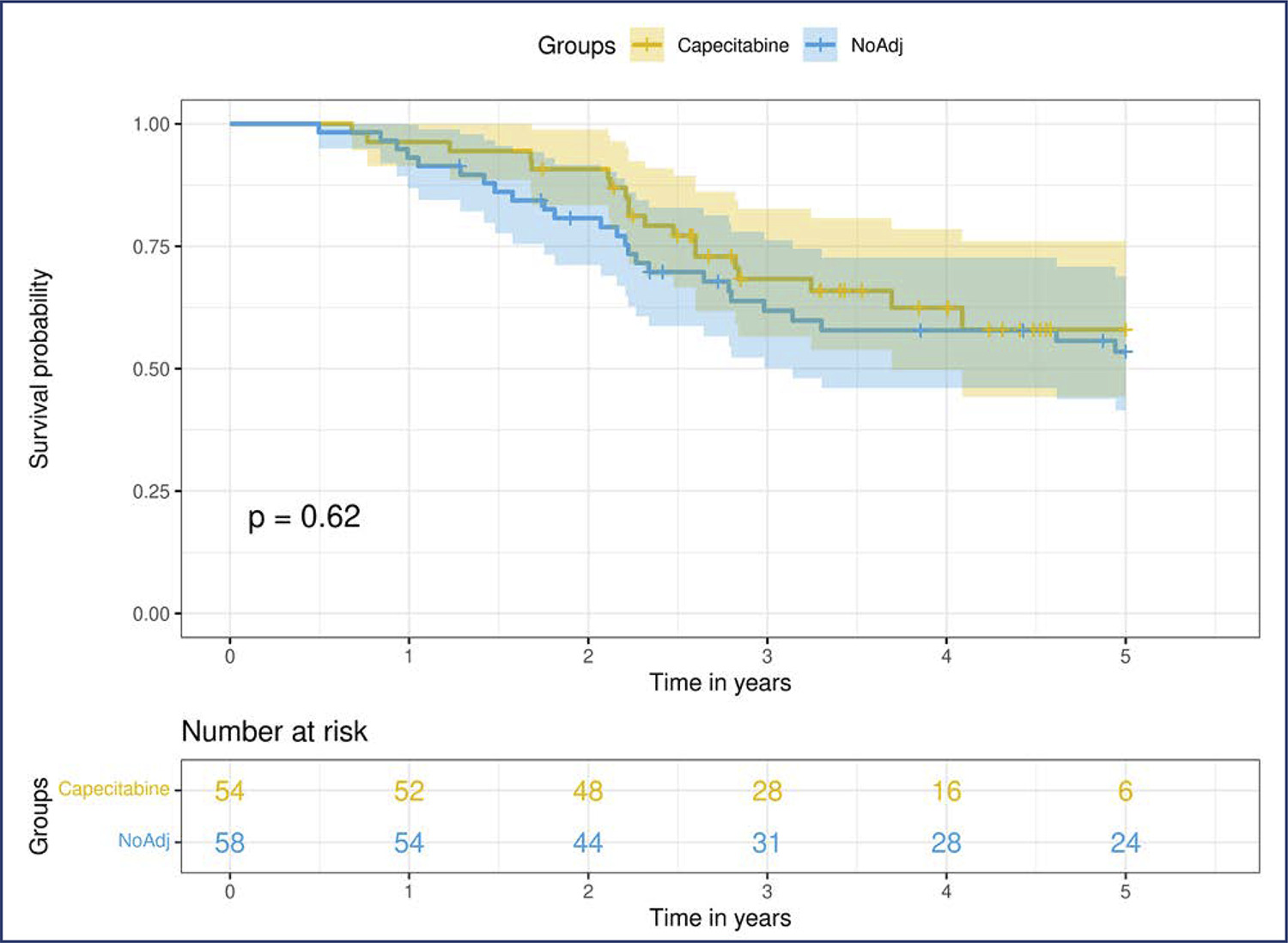
Neoadjuvant chemotherapy (NACT) has become the standard of care for patients with triple-negative breast cancer (TNBC) with tumors > 1 cm or positive axillary nodes. Pathologic complete response (pCR) has been used as an endpoint to select patients for treatment scaling. This study aimed to examine the benefit of adding adjuvant capecitabine for TNBC patients who did not achieve pCR after standard NACT in a real-world scenario.
This retrospective cohort study included all patients with TNBC who underwent NACT between 2010 and 2020. Clinicopathological data were obtained from the patient records. Univariate and multivariate analyses were conducted at the 5 years follow-up period.
We included 153 patients, more than half of whom had stage III (58.2%) and high-grade tumors (60.8%). The overall pCR rate was 34.6%, and 41% of the patients with residual disease received adjuvant capecitabine. Disease-specific survival (DSS) among the patients who achieved pCR was significantly higher (p<0.0001). Residual disease after NACT was associated with detrimental effects on DSS. In this cohort, we did not observe any survival benefit of adding adjuvant capecitabine for patients with TNBC subjected to NACT who did not achieve pCR (p=0.52).
Our study failed to demonstrate a survival benefit of extended capecitabine therapy in patients with TNBC with residual disease after NACT. More studies are warranted to better understand the indication of systemic treatment escalation in this scenario.