You searched for:"Luiz Kulay Júnior"
We found (21) results for your search.Summary
Rev Bras Ginecol Obstet. 1998;20(5):245-249
DOI 10.1590/S0100-72031998000500003
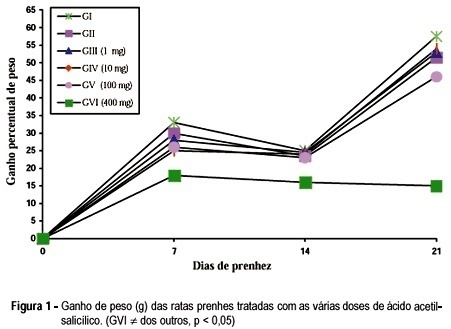
The purpose of the present study was to evaluate the effects of acetylsalicylic acid (ASA) on the pregnancy of female albino rats. We used 60 pregnant female rats which were divided into six groups of ten cache. All the animals received daily by gavage, from the 5th (day zero) until the 20th day of pregnancy, 1 ml of the following: Group I - only distilled water (control); Group II - 0.2% aqueous solution of carboxymethylcellulose (vehicle); Groups III, IV, V and VI - 1, 10, 100 and 400 mg/kg body weight respectively, of ASA diluted in 0.2% carboxymethylcellulose solution. The animals were weighed on days 0, 7, 14 and 20 of pregnancy. Our results showed that the animals treated with 100 mg of ASA presented a reduction in the number of live newborns. The animals treated with 400 mg/kg/day presented not only a reduction in the number of live newborns but also decrease in maternal, newborn and placental weight.
Summary
Rev Bras Ginecol Obstet. 2003;25(4):249-254
DOI 10.1590/S0100-72032003000400005
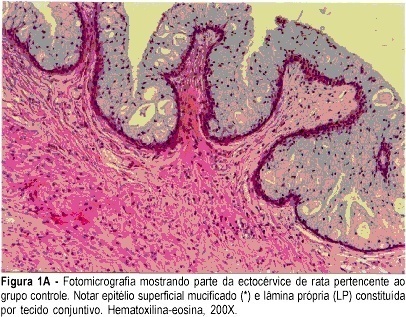
PURPOSE: to assess the morphological and morphometric alterations in the uterine cervix of pregnant albino rats determined by local hyaluronidase administration. METHODS: ten rats with a positive pregnancy test were randomly distributed into two equal groups. The control group consisted of rats that received a single dose of 1 mL distilled water in the uterine cervix, on gestational day 18, under anesthesia. The experimental group consisted of rats that received 0.02 mL hyaluronidase, diluted in 0.98 ml distilled water (total = 1 mL), in the same conditions as those of the control group. On day 20, the rats were anesthetized and submitted to dissection. The uterine cervix was prepared for morphological and morphometric study at light microscopy (hematoxylin and eosin, and Masson trichrome). RESULTS: in the experimental group, greater thinning of the superficial mucified epithelium was observed, with lamina propria rich in blood vessels and eosinophils. Diversely, the control group showed a large concentration of collagen fibers. The histometric analysis in the experimental group was characterized by a smaller number of collagen fibers (mean = 248 versus 552 of control; SD = 49.7 versus 31.1 of control). The parametric method (Student's t test) showed a significant difference between groups (p<0.0001). CONCLUSION: the local use of hyaluronidase in the cervix of pregnant rats determined predominance of loose connective tissue and a smaller concentration of collagen fibers.
Summary
Rev Bras Ginecol Obstet. 2000;22(5):281-286
DOI 10.1590/S0100-72032000000500005
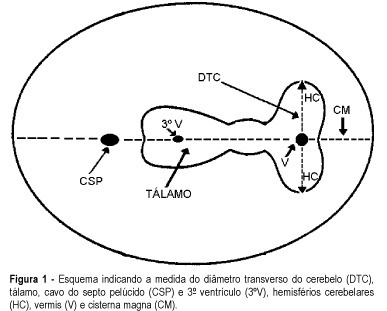
Purpose: to evaluate the effectiveness of the transverse cerebellar diameter (TCD), by ultrasonography, in the evolution of the fetal growth, and to relate it to gestational age, biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC) and femur length (FL). Method: a prospective and longitudinal study was performed on 254 pregnant women considered of low risk, with a gestational age from 20 to 40 weeks. Only 55 pregnant women were included in the study, according to inclusion and exclusion criteria. All the examinations, 217 ultrasonographic evaluations, were done by the author (LN), at least three and at most six examinations for each pregnant woman being accomplished at an interval of one to five weeks. Normality patterns were established between the 10 and 90 percentiles for each gestational age and confirmed postnatally. Results: the transverse cerebellar diameter presented a good correlation with the gestational age either as a dependent variable (R² = 0.90) or as an independent variable (R² = 0.92). A significant relationship was found in the evaluation of the fetal growth between the TCD and the several fetal parameters: BPD and HC (R² = 0.92), FL (R² = 0.90) and AC (R² = 0.89). Conclusions: the transverse cerebellar diameter is a parameter that should be used in the follow-up of development and of fetal growth because of the ascending pattern of its growth curve. Any up- or downward alteration in the growth curve can be useful for the detection of deviations of fetal growth.
Summary
Rev Bras Ginecol Obstet. 2004;26(5):349-354
DOI 10.1590/S0100-72032004000500002
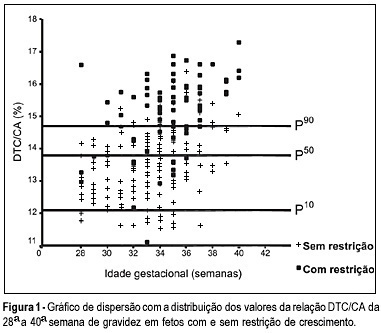
OBJECTIVE: to evaluate the accuracy of both the transverse diameter of the cerebellum (TDC) and of the transverse diameter/abdominal circumference (TDC/AC) ratio in the detection of fetal growth restriction (FGR), in high-risk pregnancies. METHOD: a prospective cross-sectional study was carried out in 260 patients with gestational age between 28 and 40 weeks. The TDC and AC of fetuses were measured through ultrasound and the fetuses with TDC below the 10th percentile or TDC/AC ratio above the 90th percentile (>14.6) were classified as FGR suspects. After birth, the accuracy of the TDC and TDC/AC was evaluated using the neonatal diagnosis of FGR as the gold standard (birth weight <10th percentile). RESULTS: after birth, 79 newborns (30.4%) were classified as small for gestational age. The TDC was appropriate in 74 (93.7%) of these fetuses and small in only 5 (6.3%). The sensitivity (SE), specificity (SP), positive (PPV) and negative (NPV) predictive values and accuracy of the TDC in the prediction of FGR were 6.3, 93.4, 29.4, 69.5, and 67%, respectively. The TDC/AC >14.6 correctly identified 59 of the 79 growth-restricted fetuses, with 27 false-positives and 20 false-negatives, SE of 74.5%, SP of 85.1%, PPV of 68.6%, NPV of 88.5% and 81.9% accuracy. CONCLUSION: the TDC is not a good screening parameter for the detection of FGR while the TDC/AC ratio above the 90th percentile is effective in this detection.
Summary
Rev Bras Ginecol Obstet. 1998;20(9):505-508
DOI 10.1590/S0100-72031998000900003
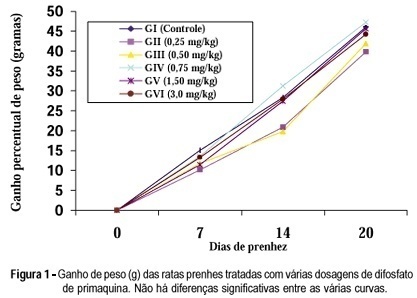
Purpose: to evaluate the chronic action of primaquine diphosphate on the pregnancy of female albino rats. Methods: sixty pregnant female rats, separated into six groups, were used. Group I received daily, by gavage, 1 ml of distilled water from day zero to the 20th day of pregnancy (control group). The female rats of the other groups also received daily, by gavage, during the same period of time the volume of 1 ml containing gradually concentrated primaquine diphosphate solution: 0.25 mg/kg, group II; 0.50 mg/kg, group III; 0.75 mg/kg, group IV; 1.5 mg/kg, group V and 3.0 mg/kg, group VI. The maternal weights were considered on day zero and on the 7th, 14th and 20th days of pregnancy, when the matrices were sacrificed. Results: the results showed that primaquine diphosphate, in the used doses, did not interfere with none of the following variables: maternal weight, newborn weight, medium individual weight of fetuses, weight of the group of placentas and medium individual weight of the placentas, implantation number, number of placentas and number of fetuses, when compared with the control group. Also there was no case of reabsorption, malformation, maternal mortality or intrauterine death, in any of the studied groups. Conclusion: in the conditions of the study there were no contraindications for the continuous use of primaquine diphosphate during the pregnancy of the female rat.