Revista Brasileira de Ginecologia e Obstetrícia. 06-01-2014;36(6):251-258
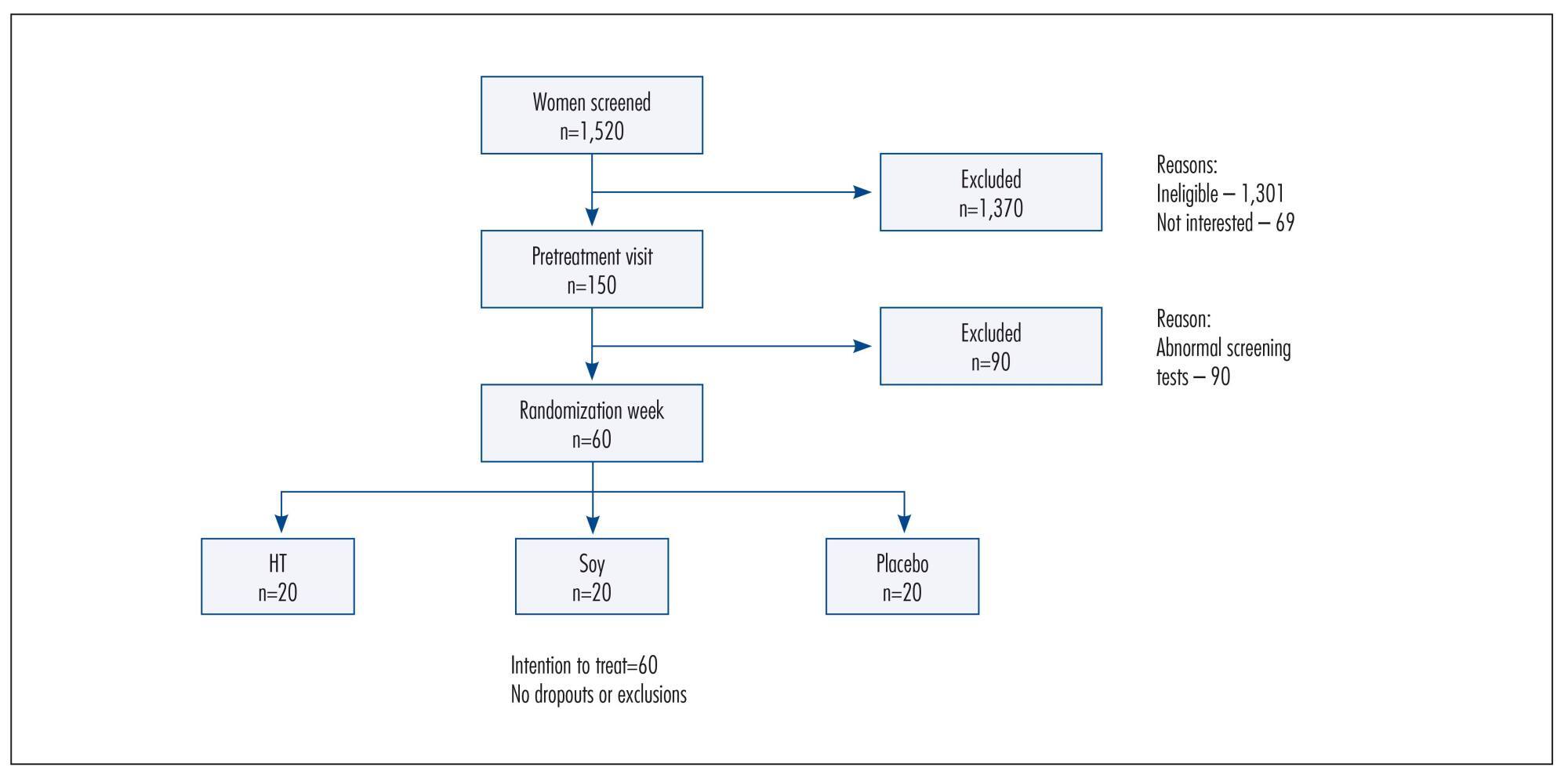
To assess the effects of a soy dietary supplement on the main biomarkers of cardiovascular health in postmenopausal women compared with the effects of low-dose hormone therapy (HT) and placebo.
Double-blind, randomized and controlled intention-to-treat trial. Sixty healthy postmenopausal women, aged 40-60 years, 4.1 years mean time since menopause were recruited and randomly assigned to 3 groups: a soy dietary supplement group (isoflavone 90mg), a low-dose HT group (estradiol 1 mg plus noretisterone 0.5 mg) and a placebo group. Lipid profile, glucose level, body mass index, blood pressure and abdominal/hip ratio were evaluated in all the participants at baseline and after 16 weeks. Statistical analyses were performed using the χ2 test, Fisher’s exact test, Kruskal-Wallis non-parametric test, analysis of variance (ANOVA), paired Student’s t-test and Wilcoxon test.
After a 16-week intervention period, total cholesterol decreased 11.3% and LDL-cholesterol decreased 18.6% in the HT group, but both did not change in the soy dietary supplement and placebo groups. Values for triglycerides, HDL-cholesterol, glucose level, body mass index, blood pressure and abdominal/hip ratio did not change over time in any of the three groups.
The use of dietary soy supplement did not show any significant favorable effect on cardiovascular health biomarkers compared with HT. Clinical Trial Registry: The trial is registered at the Brazilian Clinical Trials Registry (Registro Brasileiro de Ensaios Clínicos – ReBEC), number RBR-76mm75.
Search
Search in:


Comments