Revista Brasileira de Ginecologia e Obstetrícia. 2006;28(2):91-100
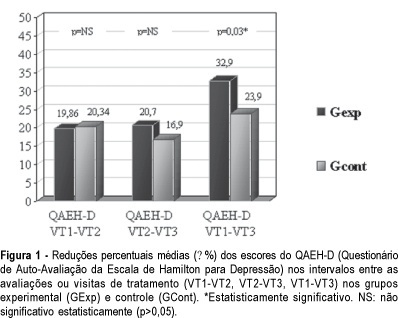
PURPOSE: to evaluate the efficacy of the use of isoflavones in the treatment of depressive symptoms in climacteric women. METHODS: placebo-controlled, randomized double-blind experimental study with 84 climacteric women who were assisted at the Lauro Wanderley University Hospital Ambulatory, in João Pessoa, Paraíba, Brazil. In the evaluation of the depressive symptoms the Self-evaluation questionnare of Hamilton’s rating scale for depresion (QAEH-D) was used in the pretreatment visit (VT1), and in the 8th (VT2) and 16th (VT3) week after treatment. The experimental group (GExp) received soy extract with isoflavones, 120 mg per day, and the control group (GCont), placebo. The comparison of the scores of the QAEH-D between the VT1, VT2 and VT3 groups constituted the primary measure of efficacy (t test, p<0.05). Secondary analysis included the estimate of the "domino hypothesis" and the clinical and laboratory evaluation of side effects. RESULTS: there was a significant reduction of the QAEH-D scores in the GExp (VT2
Search
Search in:


Comments