Revista Brasileira de Ginecologia e Obstetrícia. 01-12-2021;43(11):878-882
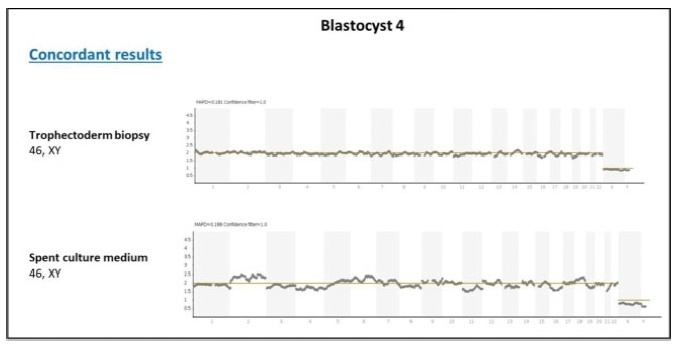
Non-invasive preimplantation genetic testing for aneuploidies (niPGT-A) aiming to assess cell-free embryonic DNA in spent culturemedia is promising, especially because it might overcome the diminished rates of implantation caused by the inadequate performance of trophectoderm (TE) biopsy. Our center is part of the largest study to date assessing the concordance between conventional PGT-A and niPGT-A, and we report here the delivery of the first baby born in Brazil using niPGT-A. The parents of the baby were admitted to our center in 2018. They did not present history of infertility, and they were interested in using in vitro fertilization (IVF) and PGT-A in order to avoid congenital anomalies in the offspring. A total of 11 (3 day-5 and 8 day-6) expanded blastocysts were biopsied, and the spent culture media (culture from day-4 to day-6) from 8 day-6 blastocysts were collected for niPGT-A. Overall, 7 embryos yielded informative results for trophectoderm (TE) and media samples. Among the embryos with informative results, 5 presented concordant diagnosis between conventional PGTA and niPGT-A, and 2 presented discordant diagnosis (1 false-positive and one falsenegative). The Blastocyst 4, diagnosed as 46, XY by both niPGT-A and conventional PGTA, was warmed up and transferred, resulting in the birth of a healthy 3.8 kg boy in February 2020. Based on our results and the recent literature, we believe that the safest current application of niPGT-A would be as a method of embryo selection for patients without an indication for conventional PGT-A. The approximate 80% of reliability of niPGT-A in the diagnosis of ploidy is superior to predictions provided by other noninvasive approaches like morphology and morphokinetics selection.
Search
Search in:


Comments