Revista Brasileira de Ginecologia e Obstetrícia. 11-01-2018;40(11):713-721
Does the use of metformin have an influence on the outcomes of preeclampsia (PE)?
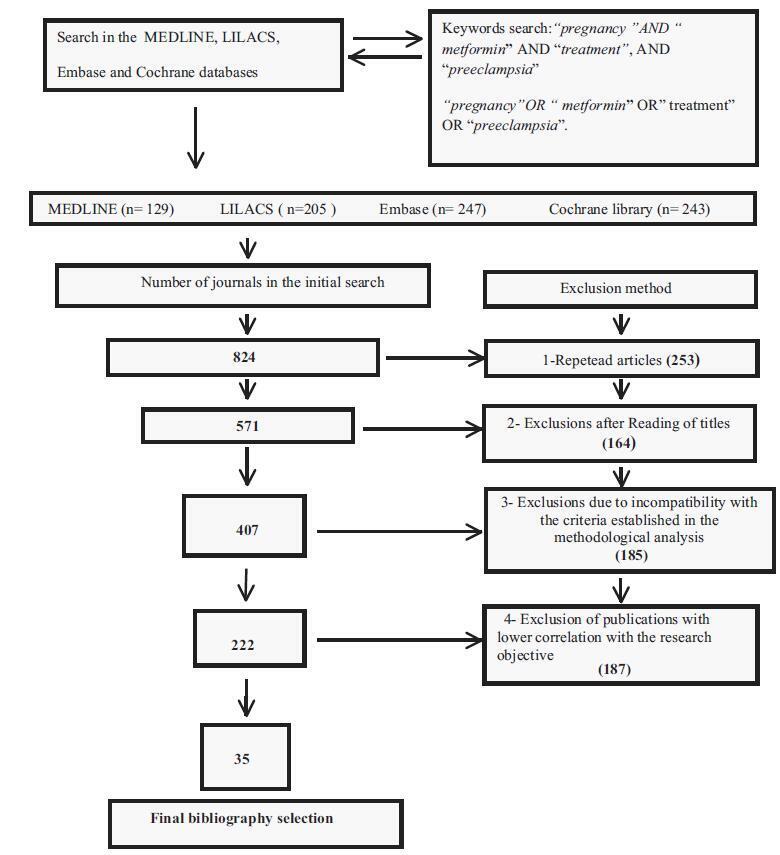
The descriptors pregnancy, metformin, treatment, and preeclampsia associated with the Boolean operators AND and OR were found in the MEDLINE, LILACS, Embase and Cochrane databases. A flowchart with exclusion criteria and inclusion strategy using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol, and eligibility criteria was used. Data were extracted regarding the type of study, the applied dosage, treatment time, segment, bias risks, and the Patient, Intervention, Comparison and Outcome (PICO) strategy to identify the quality of the study.
Total number of journals in the initial search (n= 824); exclusions from repeated articles on different search engines (n= 253); exclusions after reading the titles, when the title had no correlations with the proposed theme (n= 164); exclusions due to incompatibility with the criteria established in the methodological analysis (n= 185), exclusion of articles with lower correlation with the objective of the present study (n= 187); and final bibliographic selection (n= 35).
At first, a systematic review of the literature was performed. Subsequently, from the main selection, randomized and non-randomized trials with metformin that presented their results in absolute and relative numbers of PE outcomes were selected. The variables were treated statistically in the meta-analysis with the Review Manager software (RevMan), version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration. Denmark in the Hovedistaden region.
The study showed that metmorfin presented greater preventive effects for pregnancy-induced hypertension and was less effective for PE.
Metformin may gain place in preventive treatments for PE, once the dosages, the gestational age, and treatment time are particularly evaluated. A methodological strategy with an improved perspective of innovative and/or carefully progressive dosages during pregnancy to avoid side effects and the possibility of maternal-fetal risks is suggested.
Search
Search in:


Comments