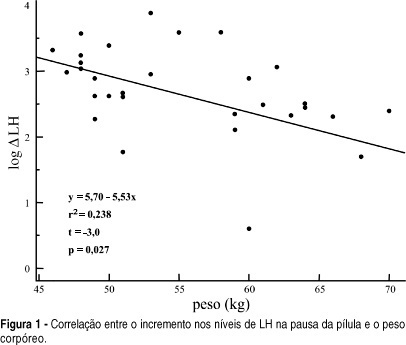
OBJECTIVE: to evaluate serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the pill-free interval of a combined oral contraceptive containing 20 mg of ethynylestradiol and 75 mg of gestodene. METHODS: thirty-one women from 17 to 36 years old, mean age of 24.5 years old, 19% adolescents, were included. FSH, LH, prolactin (PRL) and estradiol (E2) levels were measured by immunochemoluminescence. Both FSH and LH levels were measured within the last four days of pill intake and on the 7th day of the pill-free interval between two cycles. Hormonal levels were compared by the Student t-test. Comparisons between hormonal and anthropometric data were made by linear regression; values of p < 0.05 were taken as significant. RESULTS: seventy-one percent of women were using the pill for the first time. FSH levels increased from 1.3 to 5.7 mIU/ml between the end of the blister pack and the 7th day of the pill-free interval. LH increased from 0.8 to 4.3 mIU/ml. E2 levels changed from 20.2 to 28.0 pg/ml. The levels of PRL decreased from 12.4 to 10.2 ng/ml. There was no correlation between the changes in gonadotrophin levels and most of the anthropometric parameters in these women, with body mass index < 25 kg/m². CONCLUSION: the gonadotrophin levels detected on the last four days of pill intake were greatly suppressed, recovery of three to four times in amount occurring on the 7th day of the pill-free interval.
Search
Search in:


Comments