Revista Brasileira de Ginecologia e Obstetrícia. 2003;25(7):491-499
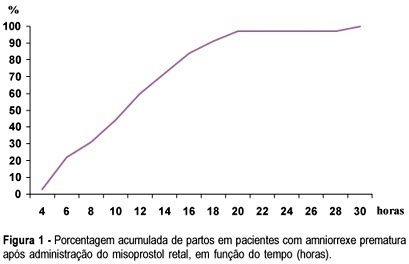
PURPOSE: to investigate whether rectally administered misoprostol is an effective method for induction of labor in patients with premature ruptured membranes at term. METHODS: a pilot trial was conducted, enrolling 32 women with alive, singleton, cephalic fetus and ruptured membranes between 36 and 41 weeks of pregnancy, with Bishop score <6 and without evidence of labor. They received rectal misoprostol (tablets of 50 mg) every 4 h until active labor was diagnosed. Patients with ruptured membranes for >18 h received antibiotics (crystalline penicillin) for prophylaxis of streptococcal infeccion. Outcomes included time from induction to labor and induction to delivery, incidence of tachysystole, mode of delivery, incidence of chorioamnionitis and neonatal outcome. Statistical analysis was performed using the public domain software Epi-Info 2002. Means and standard deviations were calculated, as well as frequency distributions. Survival analysis was performed to determine percent of deliveries according to time (hours) since the administration of the first tablet. RESULTS: the mean (±SD) induction-to-labor and induction-to-delivery intervals were 299.8±199.9 and 681±340.5 min, respectively. The frequency of tachysystole was 9.4%. About 72% of patients achieved vaginal delivery. Chorioamnionitis was diagnosed in 12.5% of the patients. Median Apgar scores at 1st and 5th min were 8 and 9, respectively. There was no case of Apgar <7 at the 5th min. Neonatal sepsis occurred in 12.5% of the neonates. CONCLUSION: induction of labor with rectal misoprostol in the setting of premature rupture of membranes was effective, with 72% of vaginal deliveries and a low rate of chorioamnionitis. These findings must be confirmed by large randomized controlled trials.
Search
Search in:


Comments