Revista Brasileira de Ginecologia e Obstetrícia. 2009;31(11):552-558
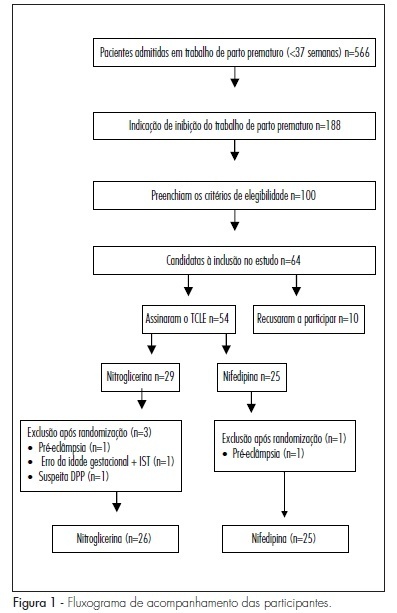
PURPOSE: to compare the effectiveness of transdermal nitroglycerin with oral nifedipine in the inhibition of preterm delivery. METHODS: a clinical essay has been performed with 50 women in preterm delivery, randomly divided into two groups, 24 receiving oral nifedipine (20 mg), and 26, transdermal nitroglycerin (10 mg patch). Patients with a single gestation, between the 24th and the 34th weeks and diagnosis of preterm delivery were selected. Women with fetal malformation and clinical or obstetric diseases were excluded. The variables analyzed were: effective tocolysis, time needed for tocolysis, recurrence frequency, progression to preterm delivery, and side effects. RESULTS: tocolysis efficacy in the first 12 hours was similar between the groups (nitroglycerin: 84.6% versus nifedipine: 87.5%; p=0.50). The time average time needed for tocolysis was also similar (6.6 versus 5.8 hours; p=0.30). There was no difference between the groups, concerning the recurrence of preterm delivery (26.9 versus 16.7%; p=0.30), and neither in the rate of preterm delivery within 48 hours (15.4 versus 12.5%; p=0.50). Nevertheless, the cephalea rate was significantly higher in the Nitroglycerin Group (30.8 versus 8.3%; p=0.04). CONCLUSIONS: transdermal nitroglycerin has presented similar effectiveness to oral nifedipine to inhibit preterm delivery in the first 48 hours, however with higher cephalea frequency.
Search
Search in:


Comments